Abstract
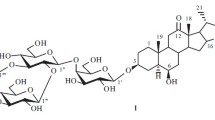
A new steroid glycoside of the spirostan series — eruboside B (I) — has been isolated from an ethanolic extract of the bulbs ofAllium erubescens C. Koh. In an acid hydrolysate, the aglycone β-chlorogenin (II) and the sugars D-glucose and D-galactose in a ratio of 3:1 have been found. By methylation, partial hydrolysis, and oxidation the structure of the spirostanol (I) has been established as (25R)-5α-spirostan-3β,6β-diol 3-O-{[O-β-D-glucopyranosyl-(1→3)]-[O-β-D-glucopyranosyl-(1→2)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside}.
Similar content being viewed by others
Literature cited
Yu. S. Vollerner, M. B. Gorovits, T. T. Gorovits, and N. K. Abubakirov, Khim. Prir. Soedin., 740 (1978).
M. E. Wall, C. R. Eddy, M. L. McClennan, and M. E. Klump, Anal. Chem.,24, 1337 (1952).
J. Romo, G. Rosenkranz, and F. Sondheimer, J. Am. Chem. Soc.,76, 5169 (1954).
L. I. Éristavi, M. B. Gorovits, and N. K. Abubakirov, Khim. Prir. Soedin., 124 (1973).
S. Hakomori, J. Biochem.,55, 205 (1964).
T. Okanishi, A. Akahori, and F. Yasuda, Chem. Pharm. Bull.,13, 545 (1965).
J. M. Van der Veen, J. Org. Chem.,28, 564 (1963).
V. V. Isakov et al., Khim. Prir. Soedin., 78 (1972).
N. K. Kochetkov et al., Chemistry of the Carbohydrates [in Russian], Moscow (1967), p. 63.
C. Sannié, S. Heitz, and H. Lapin. Compt. Rend.,233, 1670 (1951).
R. Kuhn, L. Löw, and H. Trischmann, Chem. Ber.,90, 203 (1957).
F. Kawasaki et al., Tetrahedron,21, 299 (1965).
M. B. Gorovits, A. N. Kel'ginbaev, F. S. Khristulas, and N. K. Abubakirov, Khim. Prir. Soedin., 562 (1973).
Additional information
Institute of the Chemistry of Plant Substances, Academy of Sciences of the Uzbek SSR, Tashkent. Tbilisi State Medical Institute. Translated from Khimiya Prirodnykh Soedinenii, No. 4, pp. 509–514, July–August, 1979.
Rights and permissions
About this article
Cite this article
Chincharadze, D.G., Kel'ginbaev, A.N., Gorovits, M.B. et al. Steroid saponins and sapogenins ofAllium. XV. Eruboside B fromAllium erubescens . Chem Nat Compd 15, 442–446 (1979). https://doi.org/10.1007/BF00565042
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00565042