Summary
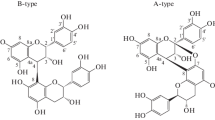
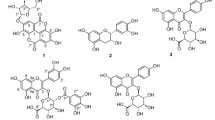
From the bark and roots ofSpiraea hypericifolia L. we have isolated and identified proanthocyanidins consisting of dimers of 3,3′,4′,5,7-pentahydroxyflavans: a dimer from the bark (B-1) with the 2R:3R configuration of the asymmetric centers of the “top” half of the molecule and 2R:3S of the “bottom” half and a dimer from the roots with the 2R:3R configurations of the asymmetric centers of both the “top” and “bottom” halves of the molecule.
Similar content being viewed by others
Literature cited
A. L. Kursanov, Biokhimiya,6, 128 (1941).
K. Freudenberg, Scient. Proc. Roy. Dublin Soc.,27, No. 4, 153 (1956).
K. Weinges, W. Bähr, W. Ebert, K. Goritz, and H. D. Mark, Fortschr. Chem. Org. Naturstoffe,27, 158 (1969).
K. Weinges, Acta Physica et Chimica; Debrecen,17, 249 (1971).
K. Weinges, W. Kaltenhauser, H. D. Marx, E. Nader, F. Nader, J. Perner, and D. Seiler, Ann. Chem.,711, 184 (1968).
R. S. Thompson, D. Jacques, E. Haslam, and R. J. V. Tanner, J. Chem. Soc., Perkin I,11, 1387 (1972).
K. Weinges, K. Göritz, and F. Nader, Ann. Chem.,715, 164 (1968).
Additional information
S. M. Kirov Kazakh State University, Alma-Ata. Translated from Khimiya Prirodnykh Soedinenii, No. 6, pp. 735–742, November–December, 1976.
Rights and permissions
About this article
Cite this article
Pashinina, L.T., Storozhenko, N.D. & Chumbalov, T.K. Proanthocyanidin dimers fromSpiraea hypericifolia . Chem Nat Compd 12, 660–665 (1976). https://doi.org/10.1007/BF00564954
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00564954