Summary
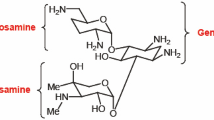
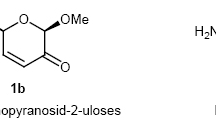
The partial synthesis of erychroside from erysimin and D-xylose has been carried out with a yield of 22%. It has been shown that the condensation of erysimin with bromoacetylxylose takes place predominantly at the C4 equatorial hydroxyl of the digitoxose residue and to a considerably smaller extent at the C3 axial hydroxyl.
Similar content being viewed by others
References
I. F. Makarevich and M. Ya. Tropp, Farmatsevt. zhurnal,4, 36, 1960.
I. F. Makarevich, M. Ya. Tropp, and D. G. Kolesnikov, DAN SSSR,136, 3, 617, 1961.
W. Königs and E. Knorr, Ber.,34, 957, 1901.
K. Reyle, K. Meyer and T. Reichstein, Helv. Chim. Acta,33, 1541, 1950.
V. T. Chernobai, ZhOKh,34, 3, 1018, 1964.
E. Fischer and M. Bergmann, Ber.,50, 1047, 1917.
W. Klyne, Biochem. J.,47, N. 4, 1950.
J. M. Webb and H. B. Levy, J. Biol. Chem.,213, 107, 1955.
A. Hunger and T. Reichstein, Helv. Chim. Acta.,35, 1073, 1952.
H. Killiani, Arch. d. Pharm.,234, 438, 1896.
Author information
Authors and Affiliations
Additional information
Khimiya Prirodnykh Soedinenii, Vol. 2, No. 6, pp. 416–420, 1966
Rights and permissions
About this article
Cite this article
Makarevich, I.F. Partial synthesis of erychroside. Chem Nat Compd 2, 341–344 (1966). https://doi.org/10.1007/BF00564219
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00564219