Conclusions
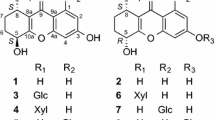
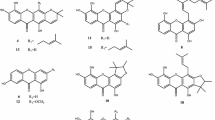
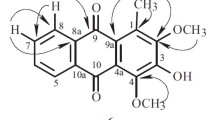
1. Xanthalin has been shown to have the structure of 2′, 2′-dimethyl-3′, 4′,-diangeloyloxy-3′, 4′-dihydropyrano-5′, 6′:6, 7-coumarin on the basis of the preparation of a number of derivatives and cleavage products.
2. The following products of the alkaline hydrolysis of xanthalin have been isolated and characterized for the first time: (±)-3′, 4′-dihydroxy-3′, 4′-dihydroxanthyletin (isokhellactone), C14H14O5, with mp 213–215° C and (−)-trans-3′-hydroxy-4′-methoxy-3′, 4′-dihydroxanthyletin (isomethylkhellactone, C15H16O5, with mp 136.5–138° C and [α] 20D −47.7 (ethanol).
Similar content being viewed by others
References
A. I. Sokolova, G. K. Nikonov, M. E. Perel'son, G. P. Syrova, and Yu. N. Sheinker, KhPS [Chemistry of Natural Compounds], 280, 1968.
E. B. Zorin, G. K. Nikonov, and G. Yu. Pek, KhPS [Chemistry of Natural Compounds], 3, 1967.
J. Lemmich, E. Lemmich, and B. E. Nielsen, Acta Chem. Send., 20, 1966.
T. R. Seshadri and M. S. Sood, Tetrah. Let., 45, 3367, 1964.
T. O. Soine and F. H. Jawad, J. Pharm. Sci., 53, 8, 990, 1964.
Author information
Authors and Affiliations
Additional information
Khimiya Prirodnykh Soedinenii, Vol. 6, No. 1, pp. 14–19, 1970
Rights and permissions
About this article
Cite this article
Sokolova, A.I., Nikonov, G.K. Structure of xanthalin. Chem Nat Compd 6, 12–15 (1970). https://doi.org/10.1007/BF00564147
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00564147