Conclusions
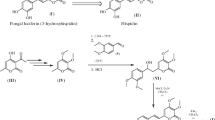
1. The synthesis of the luciferin ofLuciola mingrelica, i.e., D(−)-2-(6-hydroxybenzothiazol-2-yl)-3,4-dihydrothiazole-4-carboxylic acid, has been performed with some modifications.
2. It has been shown that in the mass spectrum of luciferin the most characteristic ions are those that correspond to decarboxylation, aromatization, and, finally, the elimination of the thiazoline part of the molecule. The spectra of the intermediate products of synthesis are characterized by competing processes of the elimination of the substituent from position 2 of the benzothiazole molecule, and, to a small extent, the “phenolic” fragmentation of the benzene ring.
3. Synthetic luciferin in combination with purified luciferase of the glow wormLuciola mingrelica can be used for the luminescence determination of the concentration of ATP in biological materials.
Similar content being viewed by others
Literature cited
C. J. Spruit, Enzymol.,13, 191 (1949).
E. H. White, F. McCapra, G. F. Field, and W. D. McElroy, J. Am. Chem. Soc.,83, 2402 (1961).
J. Walker, J. Chem. Soc., (C), 1522 (1968).
H. H. Seliger, W. D. McElroy, and E. H. White, Proc. Nat. Acad. Sci., U.S.47, 1129 (1961).
B. L. Strehler and S. R. Totter, Arch. Bioch. Bioph.,40, 28 (1952).
M. E. Ladygina, Sel'skokhoz. Biol.,2, No. 3, 416 (1967).
B. Bitler and W. D. McElroy, Arch. Bioch. Bioph.,72, 358 (1957).
E. H. White, H. Worther, H. H. Seliger, W. D. McElroy, J. Am. Chem. Soc.,88, 2015 (1966).
E. H. White, F. McCapra and G. F. Field, J. Am. Chem. Soc.,85, 337 (1963).
S. Seto, K. Ogura, Y. Wischiyama, Bull. Chem. Soc., Japan,36, 331, (1963).
E. H. White, H. Worther, G. F. Field, and W. D. McElroy, J. Org. Chem., 30, 2344 (1965).
G. P. Kukarskikh, T. E. Krendeleva, and A. B. Rubin, Biofizika17, No. 1, 85 (1972).
A. N. Kost, G. A. Golubeva, L. A. Sviridova, Khim. Prirodn. Soedin., No. 4, 495 (1973).
B. J. Millord and A. F. Temple, Organ. Mass. Spectrom.,1, 285 (1968).
G. M. Clarke, R. Grigg, and D. C. Williams, J. Chem. Soc., (B), 339 (1961).
A. Friedman, C. R. Acad. Sci., C269, 273 (1969).
H. Ogura, S. Sugimoto, and T. Ho, Organ. Mass. Spectrom.,3, 1341 (1970).
R. A. Khmel'nitskii, E. A. Kupina, S. L. Gusinskaya, and V. Yu. Tell', Khim. Prirodn. Soedin., No. 10, 1372 (1972).
M. J. Rix and B. R. Webster, Organ. Mass. Spectrom.,5, 311 (1971).
E. Barni, J. Heterocycl. Chem.,3, 501 (1972).
J. Beynon, Mass Spectrometry and Its Application to Organic Chemistry, Elsevier, Amsterdam (1960).
C. G. Stuckwisch, J. Am. Chem. Soc.,71, 4317 (1949).
O. H. Lowry, N. J. Rosebrough, A. I. Farr, and R. Randall, J. Biol. Chem.,193, 265 (1951).
S. P. Calowick and N. O. Kaplan, Methods in Enzymology, Vol. 2, Academic Press (1965), p. 598.
Additional information
M. V. Lomonosov Moscow State University. Translated from Khimiya Prirodnykh Soedinenii, No. 3, pp. 293–300, May–June, 1974.
Rights and permissions
About this article
Cite this article
Rubin, B.A., Kost, A.N., Kukarskikh, G.P. et al. Synthesis and mass-spectral study of the luciferin of Luciola mingrelica. Chem Nat Compd 10, 303–308 (1974). https://doi.org/10.1007/BF00563881
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00563881