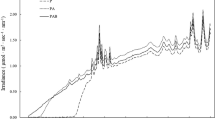
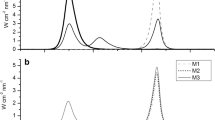
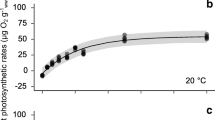
Abstract
Two vegetative clones (designated 11/85 and 7/86 in accordance with month/year of collection) of the chlorophyte macroalga Ulva rotundata were collected in the vicinity of Beaufort, North Carolina, USA. Each was grown in an outdoor continuous-flow system in summer (>-20°C) of 1986 and late winter (10° to 17°C) of 1987 in graded scalar quantum irradiances ranging from 9 to 100% of full sunlight, with and without NH +4 enrichment. The pigment content of plants from each irradiance was determined following 4 to 8 d sunny weather. Chlorophyll (chl) and carotenoid content were inverse curvilinear functions of irradiance. The chl a:b and carotenoid: chl ratios were positively related to irradiance. The close nonlinear relationship between chl (a+b) and the chl a:b ratio was independent of clone, temperature or NH +4 -enrichment. Chl (a+b) content was linearly correlated with light-regulated growth rate in the summer, but showed a marked hysteresis in the relationship in winter due to photoinhibition. The photon growth yield (PGY, i.e., the biomass yield per unit absorbed light) was maximal for plants grown at slightly subsaturating irradiances, and dropped off sharply at lower irradiances. At higher irradiances, PGY declined gradually in summer and markedly in winter. Light absorption exceeded growth needs at full sunlight, suggesting that U. rotundata was incapable of further reducing its pigment content when growth rate was light-saturated. This, along with the linear chlgrowth relationship, is consistent with photosynthetic feedback regulation of chl content. Regardless of the mechanism, chl regulation may operate within the constraints of a resource tradeoff between light harvesting and carboxylation capacities, such that pigmentation must be optimized rather than maximized.
Similar content being viewed by others
Literature cited
Alberte, R. S., Thornber, J. P. (1974) The correlation between chlorophyll a/b ratio and proportions of chlorophyll-protein complexes in green plants. Pl. Physiol. 53 (Suppl.) p. 63
Anderson, J. M. (1986). Photoregulation of the composition, function, and structure of thylakoid membranes. A. Rev. Pl. Physiol. 37: 93–136
Anderson, J. M., Osmond, C. B. (1987). Shade-sun responses: compromises between acclimation and photoinhibition. In: Kyle D.J., Osmond, C. B., Arntzen, C. J. (eds.) Photoinhibition. Elsevier, Amsterdam, p. 1–38
Beardall, J., Morris, I. (1976). The concept of light intensity adaptation in marine phytoplankton: some experiments with Phaeodactylum tricornutum. Mar. Biol. 37: 377–387
Boardman, N. K. (1977). Comparative photosynthesis of sun and shade plants. A. Rev. Pl. Physiol. 28: 355–377
Brown, J. S. (1987). Functional organization of chlorophyll a and carotenoids in the alga, Nannochloropsis saline. Pl Physiol. 83: 434–437
Chow, W. S., Anderson, J. M. (1987). Photosynthetic responses of Pisum sativum to an increase in irradiance during growth. I. Photosynthetic activities. Aust. J. Pl. Physiol. 14: 1–8
Cosper, E. (1982). Effects of diurnal fluctuations in light intensity on the efficiency of growth of Skeletonema costatum (Grev.) Cleve (Bacillariophyceae) in a cyclostat. J. exp. mar. Biol. Ecol. 65: 229–239
Davies, B. H. (1976) Carotenoids. In: Goodwin, T. W. (ed.) Chemistry and biochemistry of plant pigments. Academic Press, London, p. 38–166
Demmig, B., Winter, K., Krüger, A., Czygan, F.-C. (1987). Photoinhibition and zeaxanthin formation in intact leaves. A possible role of the xanthophyll cycle in the dissipation of excess light energy. Pl. Physiol. 84: 218–224
Demmig, B., Winter, K., Krüger, A., Czygan, F.-C. (1988). Zeaxanthin and the heat dissipation of excess light energy in Nerium oleander exposed to a combination of excess light energy and water stress. Pl. Physiol. 87: 7–24
Dubinsky, Z: (1980). Light utilization efficiency in natural phytoplankton communities. In: Falkowski, P. G. (ed.) Primary productivity in the sea. Plenum Press, New York, p. 83–97
Dubinsky, Z., Falkowski, P. G., Wyman, K. (1986). Light harvesting and utilization by phytoplankton. Pl. Cell Physiol., Kyoto 27: 1335–1349
Duke, C. S. (1985). Temporal scales of nitrogen availability and seaweed physiological response. Ph.D. dissertation. Duke University, Durham, North Carolina
Duke, C.S., Lapointe, B. E., Ramus, J. (1986). Effects of light on growth, RuBPCase activity and chemical composition of Ulva species (Chlorophyta). J. Phycol. 22: 362–370
Duysens, L. N. M. (1956). The flattening of the absorption of suspensions, as compared to that of solutions. Biochim. biophys. Acta 19: 1–12
Evans, J. R. (1986). Photosynthesis and nitrogen partitioning in leaves of T. aestivum and related species. Ph.D. thesis Australian National University, Canberra
Evans, J. R. (1986). A quantitative analysis of light distribution between the two photosystems, considering variation in both the relative amounts of the chlorophyll-protein complexes and the spectral quality of light. Photobiochem. Photobiophys. 10: 135–148
Falkowski, P. G. (1980) Light-shade adaptation in marine phytoplankton. In: Falkowski, P. G. (ed.) Primary productivity in the sea. Plenum Press, New York, p. 99–119
Falkowski, P. G., Dubinsky, Z., Wyman, K. (1985). Growth-irradiance relationships in phytoplankton. Limnol. Oceanogr 30: 311–321
Geider, R. J., Osborne, B. A. (1986). Light absorption, photosynthesis and growth of Nannochloris atomus in nutrient-saturated cultures. Mar. Biol. 93: 351–360
Geider, R. J., Osborne, B. A., Raven, J. A. (1985). Light dependence of growth and photosynthesis in Phaeodactylum tricornutum (Bacillariophyceae). J. Phycol. 21: 609–619
Henley, W. J., Ramus, J. (1989a). Photoacclimation of Ulva rotundata (Chlorophyta) under natural irradiance. Mar. Biol. 103: 261–266
Henley, W.J., Ramus, J. (1989b). Photoacclimation and growth rate responses of Ulva rotundata (Chlorophyta) to intraday variations in growth irradiance. J. Phycol. 25: 398–401
Henley, W. J., Ramus, J. (1989c). Time course of physiological response of Ulva rotundata to growth irradiance transitions. Mar. Ecol. Prog. Ser. 54: 171–177
Kiefer, D. A., Mitchell, B. G. (1983). A simple, steady state description of phytoplankton growth based on absorption cross section and quantum efficiency. Limnol. Oceanogr. 28: 770–776
Kirk, J. T. O. (1983). Light and photosynthesis in aquatic ecosystems. Cambridge University Press, Cambridge
Lapointe, B. E. (1981). The effects of light and nitrogen on growth, pigment content, and biochemical composition of Gracilaria foliifera v. angustissima (Gigartinales, Rhodophyta). J. Phycol. 17: 90–95
Lapointe, B. E., Dawes, C. J., Tenore, K. R. (1984a). Interactions between light and temperature on the physiological ecology of Gracilaria tikvahiae (Gigartinales: Rhodophyta). II. Nitrate uptake and levels of pigments and chemical constitutents. Mar. Biol. 80: 171–178
Lapointe, B. E., Duke, C. S. (1984). Biochemical strategies for growth of Gracilaria tikvahiae (Rhodophyta) in relation to light intensity and nitrogen availability. J. Phycol. 20: 488–495
Lapointe, B. E., Tenore, K. R. (1981). Experimental outdoor studies with Ulva fasciata Delile. I. Interaction of light and nitrogen on nutrient uptake, growth, and biochemical composition. J. exp. mar. Biol. Ecol. 53: 135–152
Lapointe, B. E., Tenore, K. R., Dawes, C. J. (1984b). Interactions between light and temperature on the physiological ecology of Gracilaria tikvahiae (Gigartinales: Rhodophyta). I. Growth, photosynthesis and respiration. Mar. Biol. 80: 161–170
Laws, E. A., Bannister, T. T. (1980). Nutrient- and light-limited growth of Thalassiosira fluviatilis in continuous culture, with implications for phytoplankton growth in the ocean. Limnol. Oceanogr. 25: 457–473
Leong, T.-Y., Anderson, J. M. (1984). Adaptation of the thylakoid membranes of pea chloroplasts to light intensities. I. Study on the distribution of chlorophyll-protein complexes. Photosynthesis Res. 5: 105–115
Levavasseur, G. (1985). Plasticite de l'appareil pimentaire des grandes algues marines. Regulations en fonction de leur environment. Thèse Doctorat. Université Pierre et Marie Curie
Lichtenthaler, H. K., Prenzel, U., Douce, R., Joyard, J. (1981). Localization of prenylquinones in the envelope of spinach chloroplasts. Biochim. biophys. Acta 641: 99–105
Lüning, K., Dring, M. J. (1985). Action spectra and spectral quantum yield of photosynthesis in marine macroalgae with thin and thick thalli. Mar. Biol. 87: 119–129
Moran, R. (1982). Formulae for determination of chlorophyllous pigments extracted with N,N-dimethylformamide. Pl. Physiol. 69: 1376–1381
Osborne, B. A., Raven, J. A. (1986). Light absorption by plants and its implications for photosynthesis. Biol. Rev. 61: 1–61
Osmond, C. B. (1987). Photosynthesis and carbon economy of plants. New Phytol. 106 (Suppl.): 161–175
Post, A. F., de Wit, R., Mur, L. R. (1985). Interactions between temperature and light intensity on growth and photosynthesis of the cyanobacterium Oscillatoria agardhii. J. Plankton Res. 7: 487–495
Ramus, J. (1978). Seaweed anatomy and photosynthetic performance: the ecological significance of light guides, heterogeneus absorption and multiple scatter. J. Phycol. 14: 352–362
Ramus, J. (1983). A physiological test of the theory of complementary chromatic adaptation. II. Brown, green and red seaweeds. J. Phycol. 19: 173–178
Ramus, J., Beale, S. I., Mauzerall, D. (1976a). Correlation of changes in pigment content with photosynthetic capacity of seaweeds as a function of water depth. Mar. Biol. 37: 231–238
Ramus, J., Beale, S. I., Mauzerall, D., Howard, K. L. (1976b). Changes in photosynthetic pigment concentration in seaweeds as a function of water depth. Mar. Biol. 37: 223–229
Ramus, J., Lemons, F., Zimmerman, C. (1977). Adaptation of light-harvesting pigments to downwelling light and the consequent photosynthetic performance of the eulittoral rockweeds Ascophyllum nodosum and Fucus vesiculosus. Mar. Biol. 42: 293–303
Raven, J. A. (1984). A cost-benefit analysis of photon absorption by photosynthetic unicells. New Phytol. 98: 593–625
Raven, J. A., Beardall, J. (1982). The lower limit of photon fluence rate for phototrophic growth: the significance of ‘slippage’ reactions. Pl. Cell Envir. 5: 117–124
Richardson, K., Beardall, J., Raven, J. A. (1983). Adaptation of unicellular algae to irradiance: an analysis of strategies. New Phytol. 93: 157–191
Senger, H., Fleischhacker, P. (1978). Adaptation of the photosynthetic apparatus of Scenedesmus obliquus to strong and weak light conditions. Physiologia Pl. 43: 35–42
Shuter, B. (1979). A model of physiological adaptation in unicellular algae. J. theor. Biol. 78: 519–552
Siefermann-Harms, D. (1985). Carotenoids in photosynthesis. I. Location in photosynthetic membranes and light-harvesting function. Biochim. biophys. Acta 811: 325–355
Steele, J. H. (1962). Environmental control of photosynthesis in the sea. Limnol. Oceanogr. 7: 137–150
Sukenik, A., Bennett, J., Falkowski, P. G. (1988). Changes in the abundance of individual apoproteins of light-harvesting chlorophyll a/b-protein complexes of phtosystem I and II with growth irradiance in the marine chlorophyte, Dunaliella tertiolecta. Biochim. biophys. Acta 932: 206–215
Talling, J. F. (1957). Photosynthetic characteristics of some freshwater plankton in relation to underwater radiation. New Phytol. 56: 29–50
Webber, A. N., Heath, R. L., Frederick, P. E., Thomson, W. W. (1986). Marginal regions of thylakoid membranes. Pl. Physiol. 80 (Suppl.): p. 146
Wyman, M., Gregory, R. P. F., Carr, N. G. (1985). Novel role for phycoerythrin in a marine cyanobacterium, Synechococcus Strain DC2. Science, N.Y. 230: 818–820
Author information
Authors and Affiliations
Additional information
Communicated by J. M. Lawrence, Tampa
Rights and permissions
About this article
Cite this article
Henley, W.J., Ramus, J. Optimization of pigment content and the limits of photoacclimation for Ulva rotundata (Chlorophyta). Mar. Biol. 103, 267–274 (1989). https://doi.org/10.1007/BF00543357
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00543357