Abstract
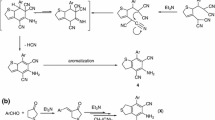
An ester of p-nitrobenzoylacetic acid is cyclized under the action of hexamethyl-enetetramine and ammonium acetate to 3,5-diethoxycarbonyl-3-p-nitrobenzoyl-6-p-nitrophenyl-1,2,3,4-tetra-hydropyridine, the structure of which has been established by NMR, UV, IR, and mass spectra and also by its chemical reactions.
Similar content being viewed by others
Literature cited
B. S. Chekavichus, A. E. Sausin', R. M. Zolotoyabko, and G. Ya. Dubur, Izv. Akad. Nauk LatvSSR, Ser. Khim., No. 1, 77 (1985).
Ch. Pigerol, N. N. Chandavoine, and P. de Cointet de Fillain, W. Ger. Patent 2,844,782; Chem. Abs., 91, 58137v (1979).
W. Bremser, B. Franke, and A. Wagner, Chemical Shift Range in Carbon-13 NMR Spectroscopy, Verlag Chemie, Basel (1982), p. 59.
D. Hofmann, E. M. Kosower, and K. Wallenfels, J. Am. Chem. Soc., 83, 3314 (1961).
J. V. Greenhill, J. Chem. Soc., No. 15, 2699 (1971).
H. Möhrle, S. Dörnbrack, and H. Novak, Monatsh. Chem., 112, 1417 (1981).
J. Martens, K. Praefcke, and H. Schwartz, Z. Naturforsch, Teil B, 30b, 259 (1975).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 4, pp. 501–505, April, 1986.
Rights and permissions
About this article
Cite this article
Chekavichus, B.S., Sausin', A.E., Zolotoyabko, R.M. et al. Formation of a derivative of 3,3,5-tricarbonyl-1,2,3,4-tetrahydropyridine under the conditions of the hantzsch synthesis. Chem Heterocycl Compd 22, 410–414 (1986). https://doi.org/10.1007/BF00542780
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00542780