Abstract
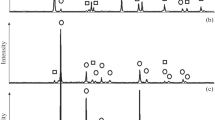
The growth curves for TiN formed from four anatases and two rutiles by carbothermal reduction in nitrogen at 1090–1230 °C were analysed in terms of several different mathematical models. The results for all samples over the complete temperature range are best described by first-order kinetics. Arrhenius plots show evidence of two lines intersecting at about 1100 °C. Above this temperature, the activation enthalpies for the anatases and rutiles fall in the ranges 278–392 and 263–264 kJ mol−1 respectively, and the activation entropies are all of negative sign. Below 1100 °C, the activation enthalpies are about 714–971 kJ mol−1, and the activation entropies are all of positive sign. Comparison of the amounts of TiN formed at long reaction times with thermodynamic calculations of the equilibrium phase composition at each temperature suggests that the reactant powder bed experiences a nitrogen concentration substantially in excess of the stoichiometric requirement; diffusion of the gas phase into the bed therefore appears not to be the rate-limiting step. The first-order kinetics, and their virtual independence of the physical and chemical properties of the reactants probably reflect the complexity of the reaction sequence.
Similar content being viewed by others
References
G. V. White, K. J. D. Mackenzie and J. H. Johnston, J. Mater. Sci. 27 (1992) 4287.
G. V. White, K. J. D. Mackenzie, I. W. M. Brown, M. E. Bowden and J. H. Johnston, ibid. 27 (1992) 4294.
G. V. Samsonov and C. S. Polishchuk, J. Appl. Chem. USSR 46 (1973) 257.
J. H. Sharp, G. W. Brindley and B. N. Narahariachar, J. Amer. Ceram. Soc. 49 (1966) 379.
K. J. D. Mackenzie, Trans. J. Brit. Ceram. Soc. 74 (1975) 121.
G. D. Bogmolov, V. D. Lyubimov and G. P. Shveikin, Zh. Prikl. Khim. 44 (1971) 1205.
V. D. Lyubimov, T. V. Shestakova, G. P. Shveikin and S. I. Alyamovskii, Russ. J. Inorg. Chem. 22 (1977) 1620.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
White, G.V., Mackenzie, K.J.D., Brown, I.W.M. et al. Carbothermal synthesis of titanium nitride. J Mater Sci 27, 4300–4304 (1992). https://doi.org/10.1007/BF00541556
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00541556