Abstract
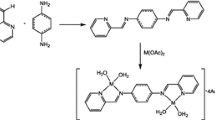
Ascheme for formation of 6,6′,7,7′-tetrahydro-2,2′,4,4′-tetraaryl-8,8′-bis(5H-1-benzothiopyranyls) through intermediate radicals and cation radicals was substantiated as a result of a study of the electrochemical behavior of 2,4-diarylcylohexa[b]thiopyrylium tetrafluoroborates and 2,4-diaryl-4H-cyclohexa[b]thiopyrans and comparison with the results of their oxidative dimerization. The formation of 2,4-diarylcyclohexa[b]thiopyranyl radicals is also confirmed by EPR spectroscopy.
Similar content being viewed by others
Literature cited
S. K. Klimenko, N. N. Ivanova, and L. M. Yudovich, Khim. Geterotsikl. Soedin., No. 1, 26 (1992).
N. T. Berberova, G. N. Dorofeenko, and O. Yu. Okhlobystin, Khim. Geterotsikl. Soedin., No. 3, 318 (1977).
I. M. Sosonkin, A. N. Domarev, N. I. Kozhevnikova, and V. G. Kharchenko, Khim. Geterotsikl. Soedin., No. 3, 318 (1984).
M. Farcasiu and D. Farcasiu, Chem. Ber., 102, 2294 (1969).
M. V. Nekhoroshev, V. B. Panov, A. A. Bumber, and O. Yu. Okhlobystin, Zh. Obshch. Khim., 50, 958 (1980).
N. T. Berberova, A. A. Bumber, M. V. Nekhoroshev, V. B. Panov, and O. Yu. Okhlobystin, Dokl. Akad. Nauk SSSR, 246, 108 (1979).
G. P. Barchan, G. A. Abakumov, A. S. Kutsarov, A. S. Morkovnik, V. M. Cherkasov, and O. Yu. Okhlobystin, Dokl. Akad. Nauk SSSR, 229, 348 (1976).
V. G. Kharchenko, S. K. Klimenko, M. N. Berezhnaya, and I. Ya. Evtushenko, Zh. Org. Khim., 10, 1302 (1974).
V. G. Kharchenko, S. K. Klimenko, M. N. Berezhnaya, and S. N. Chalaya, USSR Author's Certificate No. 447,041; Byull. Izobret., No. 10, 172 (1976).
S. K. Klimenko, T. I. Tyrina, and N. N. Sorokin, Khim. Geterotsikl. Soedin., No. 5, 614 (1987).
S. K. Klimenko, N. N. Ivanova, and N. N. Sorokin, Zh. Org. Khim., 23, 2019 (1987).
S. K. Klimenko, M. N. Berezhnaya, and V. G. Kharchenko, Zh. Org. Khim., 10, 2425 (1974).
S. K. Klimenko, T. V. Stolbova, T. I. Tyrina, I. N. Sorokin, I. F. Leshcheva, I. M. Sergeev, and V. G. Kharchenko, Khim. Geterotsikl. Soedin., No. 7, 898 (1984).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 3, pp. 349–354, March, 1992.
Rights and permissions
About this article
Cite this article
Ivanova, N.N., Berberova, N.T., Archegova, A.S. et al. Redox properties of 2,4-diaryl-substituted cyclohexa[b]thiopyrylium salts. Chem Heterocycl Compd 28, 292–296 (1992). https://doi.org/10.1007/BF00529371
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00529371