Abstract
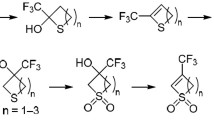
Depending on the degree of substitution of the thiopyran ring, either oxidation at the sulfur atom, which leads to the formation of the 1,1-dioxide, or dehydrogenation of the heteroring in the C(4) position may occur in the reaction of 4H-thiopyrans with hydrogen peroxide in acetic acid. In the case of dehydrogenation the composition of the products depends on the nature of the starting 4H-thiopyran. Some peculiarities of the structure of 4H-thiopyrans that promote their conversion to 1,1-dioxides were ascertained.
Similar content being viewed by others
Leterature cited
J. Suld and C. Price, J. Am. Chem. Soc., 84, 2090 (1962).
J. Suld and C. Price, J. Am. Chem. Soc., 84, 2094 (1962).
V. G. Kharchenko and V. I. Kleimenova, Zh. Org. Khim., 7, 613 (1971).
V. V. Puchkova, E. N. Gur'yanova, V. G. Kharchenko, and A. A. Rassudova, Zh. Org. Khim., 9, 1531 (1973).
V. G. Kharchenko, N. I. Kozhevnikova, S. N. Chalaya, L. G. Chichenkova, and N. N. Ivanova, Khim. Geterotsikl. Soedin., No. 3, 405 (1981).
A. I. Tolmachev, L. M. Shulezhko, and M. Yu. Kornilov, Ukr. Khim. Zh., 40, 287 (1974).
A. S. Batsanov, Yu. T. Struchkov, and L. Yu. Ukhin, Inorg. Chim. Acta, 63, 17 (1982).
M. Yu. Kornilov, L. M. Shulezhko, and A. I. Tolmachev, Teor. Éksp. Khim., 10, 508 (1974).
A. A. Shcherbakov, G. G. Aleksandrov, Yu. T. Struchkov, and V. G. Kharchenko, Khim. Geterotsikl. Soedin., No. 11, 1470 (1979).
I. Ya. Evtushenko, S. K. Klimenko, B. I. Ionin, and V. G. Kharchenko, Zh. Org. Khim., 11, 2417 (1975).
E. L. Eliel, Chem. Ind., 568 (1959).
S. K. Klimenko, V. G. Kharchenko, and T. V. Stolbova, Khim. Geterotsikl. Soedin., No. 1, 3 (1978).
V. G. Kharchenko and S. K. Klimenko, Khim. Geterotsikl. Soedin., No. 4, 630 (1967).
I. Degani, F. Taddei, and C. Vincenzi, Bull. Sci. Fac. Chim. Ind. Bologna, 25, 61 (1967).
V. G. Kharchenko, V. I. Kleimenova, and A. R. Yakoreva, Khim. Geterotsikl. Soedin., No. 7, 900 (1970).
V. G. Kharchenko and N. I. Kozhevnikova, Khim. Geterotsikl. Soedin., No. 2, 200 (1983).
V. G. Kharchenko, N. I. Kozhevnikova, L. L. Kulikova, and N. V. Voronina, Khim.-farm. Zh., No. 11, 38 (1981).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 8, pp. 1042–1046, August, 1985.
Rights and permissions
About this article
Cite this article
Kozhevnikova, N.I., Kharchenko, V.G. Effect of substituents on the nature of the products of oxidation of 4H-thiopyrans. Chem Heterocycl Compd 21, 868–871 (1985). https://doi.org/10.1007/BF00519811
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00519811