Abstract
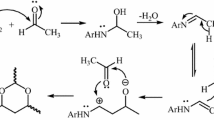
Organoaluminum compounds react with 4-methyl-2-phenyl-1,3-dioxane with cleavage of the O1-C2 bond to give monoethers with a primary alcohol group.
Similar content being viewed by others
Literature cited
L. N. Zakharkin and I. M. Khorina, Izv. Akad. Nauk SSSR, Ser. Khim., 6, 2255 (1959).
W. Zajac and K. Byrne, J. Org. Chem., 38., 384 (1973).
B. A. Trofimov and S. E. Korostova, Usp. Khim., 44, 75 (1975).
Takahiro Kosokawa, Chem. Commun., 1245 (1983).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 8, pp. 1036–1037, August, 1985.
Rights and permissions
About this article
Cite this article
Volkov, A.A., Zlot-skii, S.S., Kravets, É.K. et al. Regiospecific cleavage of 1,3-dioxanes by organoaluminum compounds. Chem Heterocycl Compd 21, 861–863 (1985). https://doi.org/10.1007/BF00519809
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00519809