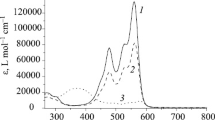
Abstract
1,3,3-Trimethylspiro(indoline-2,2'-[2H]chromenes) with CH3 and CH3O groups in the 4–7 positions and NO2 and CH3O groups in the 6′ and 8′ positions were obtained by the reaction of 4,6-, 4,7-, 5,6-, and 6,7-dimethoxy- and 4-methyl-7-methoxy-2-methyleneindolines and 1,3,3,4,5,6,7-heptamethyl-2-methyleneindoline with 3- and 5-nitrosalicylaldehydes and their derivatives. Most of the compounds have photochromic properties. The introduction of electron-donor groups into the indoline fragment of the spirochromene molecules changes the rate of the dark reaction within the limits of one order of magnitude and has a small effect on the position of the long-wave absorption band of the photomerocyanine.
Similar content being viewed by others
Literature cited
R. S. Bertelson, in: Photoehromism, G. Brown, ed., Wiley Interscience, New York (1971), p. 45.
Ishii Hisashi, Murokami Yasuoki, Tani Syohai, Abe Kadzuya, and Ikeda Hisaburo, J. Pharm. Soc. Jpn., 90, 724 (1970); Ref. Zh. Khim., 4Zh365 (1971).
G. Plancher, Chem. Ber., 31, 1488 (1898).
H. Franzen and M. Schmidt, J. Prakt. Chem., [2], 96, 1 (1917).
I. J. Delucia, J. W. Dehn, and R. A. Pazzarello, US Patent No. 3366619; Chem. Abstr., 68, 96797 (1968).
D. Bertazzoni, B. Bortoletti, and T. Berloto, Boll. Chim.-farm., 109, 60 (1970); Ref. Zh. Khim., 17Zh357 (1970).
D. Ockender and K. Schofield, J. Chem. Soc., No. 7, 3175 (1957).
M. A. Gal'bershtam, N. M. Przhiyalgovskaya, O. R. Khrolova, I. B. Lazarenko, G. K. Bobyleva, and N. N. Suvorov, Khim. Geterotsikl. Soedin., No. 12, 1640 (1977).
V. I. Pantsyrnyi and M. A. Gal'bershtam, Khim. Geterotsikl. Soedin., No. 5, 659 (1973).
M. A. Gal'bershtam, N. M. Przhiyalgovskaya, O. R. Khrolova, I. V. Manakova, G. K. Bobyleva, and N. N. Suvorov, Khim. Geterotsikl. Soedin., No. 8, 1069 (1977).
M. A. Gal'bershtam, O. R. Khrolova, G. K. Bobyleva, Yu. B. Pod'yachev, N. P. Samoilova, V. M. Bulgakov, and Yu. V. Zasukhin, Khim. Vys. Energ., 13, 230 (1979).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 9, pp. 1220–1224, September, 1982.
Rights and permissions
About this article
Cite this article
Gal'bershtam, M.A., Bondarenko, E.M., Lavrishcheva, L.N. et al. Synthesis and photochromic properties of indolinospirochromenes with electron-donor substituents in the indoline part of the molecule. Chem Heterocycl Compd 18, 937–941 (1982). https://doi.org/10.1007/BF00513436
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00513436