Abstract
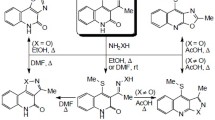
3-Acetyl-4-methylaminopyridine was obtained by cyanation of 3-acetylpyridine ethyleneketal N-oxide and subsequent chemical transformations of the nitrile group. Condensation of this product with oxindole led to the synthesis of 5H-5,11-dimethylindolo [3,2-j]-1,4-naphthyridine — the aza analog of the alkaloid ellipticine.
Similar content being viewed by others
Literature cited
N. N. Pogodaeva, V. V. Kononova, and A. A. Semenov, Khim.-Farm. Zh. (in press).
S. Goodwin, A. F. Smitz, and E. C. Horning, J. Am. Chem. Soc., 81, 1903 (1959).
A. A. Semenov, Natural Antitumorigenic Compounds [in Russian], Nauka, Novosibirsk (1979), p. 19.
M. Sainsbury, Synthesis, 7, 437 (1977).
W. Borsche, M. Wagner-Roemmich, and J. Barthenheier, Lieb. Ann., 550, 160 (1942).
G. W. Gribble, J. Org. Chem., 38, 4074 (1973).
G. B. Bachman and R. S. Barker, J. Org. Chem., 14, 97 (1949).
Author information
Authors and Affiliations
Additional information
See [1] for communication 13.
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 9, pp. 1211–1214, September, 1982.
Rights and permissions
About this article
Cite this article
Kononova, V.V., Semenov, A.A. Carbolines. 14. Synthesis of 5-azaellipticine. Chem Heterocycl Compd 18, 929–932 (1982). https://doi.org/10.1007/BF00513434
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00513434