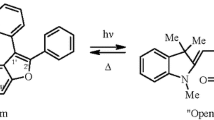
Abstract
The self-consistent-field (SCF) molecular orbital (MO) Pariser-Parr-Pople (PPP) methods within the Dewar σ,π parametrization for the calculation of the properties of the ground states and variable β for the description of the excited states were used to estimate the relative stabilities of the spiropyran forms in spiranmerocyanine valence-tautomeric equilibria and to predict the electronic spectra of the photocolored and thermocolored forms of spirans. A correlation was found between the energies of anionic localization of the heterocation and the electron charge in the hypothetical position of spirocyclization and the experimental data on the stability of the spiropyran form. Another correlation links the calculated differences in the energies of the pyran and quinoneallide systems with the tendency of the latter to undergo cyclization. The stabilities of a large number of spiropyrans with different structures are predicted on the basis of the correlations obtained. The effect of various structural factors on the absorption spectra of the merocyanine forms of spiropyrans was studied. 2H-Naphtho[2,3-b]-pyran systems are recommended as promising photochromic compounds.
Similar content being viewed by others
Literature cited
V. A. Anisimova, A. F. Pozharskii, L. E. Nivorozhkin, and V. I. Minkin, Khim. Geterotsikl. Soedin., No. 1, 108 (1978).
R. C. Bertelson, in: Photochromism (ed. by G. H. Brown), Wiley-Interscience, New York (1971), Chap. III, p. 45–432.
J. Koszar, Light-Sensitive Systems, Wiley, New York (1965), Chap. II.
V. A. Barachevskii, M. A. Gal'bershtam, Yu. E. Gerasimenko, and Yu. N. Gerulaitis, Zh. Vses. Khim. O-va., 19, 85 (1974).
E. Fischer, Fortschr. Chem. Forsch., 7, 605 (1967).
V. A. Barachevskii, Zh. Vses. Khim. O-va., 19, 423 (1974).
C. Schiele, M. Ruch, and D. Hendriks, Tetrahedron, 23, 3733 (1967).
E. B. Knott, J. Chem. Soc., 3038 (1951).
V. P. Martynova, N. E. Shelepin, N. S. Loseva, É. R. Zakhs, L. E. Nivorozhkin, and V. I. Minkin, Khim. Geterotsikl. Soedin., No. 2, 167 (1971).
É. R. Zakhs, L. S. Éfros, and E. V. Bashut-skaya, Khim. Geterotsikl. Soedin., No. 11, 1580 (1973).
B. Ya. Simkin, V. I. Minkin, and L. E. Nivorozhkin, Khim. Geterotsikl. Soedin., No. 1, 76 (1974).
A. M. Samat, R. J. Guglielmetti, and G. J. Martin, Org. Magn. Res., 8, 62 (1976).
K. V. Veksler, É. R. Zakhs, V. M. Treiger, and L. S. Éfros, Khim. Geterotsikl. Soedin., No. 4, 447 (1971).
J. E. Lohr and C. Kortum, Ber. Bunsengesellsch., 70, 817 (1966).
N. E. Shelepin, Master's Dissertation, Rostov-on-Don (1972).
N. D. Dmitrieva, I. L. Belaits, R. M. Liberzon, T. D. Platonova, and Yu. E. Gerasimenko, Zh. Org. Khim., 11, 1319 (1975).
É. R. Zakhs, V. P. Martynova, and L. S. Éfros, Khim. Geterotsikl. Soedin., No. 6, 750 (1974).
L. S. Éfros, É. R. Zakhs, and N. K. Beresneva, Khim. Geterotsikl. Soedin., No. 7, 1004 (1970); No. 7, 961 (1971).
N. A. Voloshin, N. E. Shelepin, and V. I. Minkin, USSR Inventor's Certificate No. 518491 (1976); Byul. Izobr., No. 23, 75 (1976).
N. A. Voloshin, Master's Dissertation, Rostov-on-Don (1977).
L. E. Nivorozhkin, N. S. Loseva, and V. I. Minkin, Khim. Geterotsikl. Soedin., No. 3, 318 (1972).
P. Appriou and R. J. Guglielmetti, Compt. Rend., 275C, 57, 1549 (1972).
P. Appriou, F. Garnier, and R. Guglielmetti, Helv. Chim. Acta, 58, 2563 (1975).
B. S. Luk'yanov, Yu. I. Ryabukhin, G. N. Dorofeenko, L. E. Nivorozhkin, and V. I. Minkin, Khim. Geterotsikl. Soedin., No. 2, 161 (1978).
K. Sato and M. Okazaki, J. Chem. Soc. Jpn., Chem. Ind. Chem., No. 12, 2146, 2150 (1975).
H. Baumann, Ann., 717, 124 (1968).
V. I. Minkin, V. A. Kosobut-skii, B. Ya. Simkin, Yu. A. Zhdanov, J. Mol. Struct., 24, 237 (1975).
É. R. Zakhs, E. V. Bashut-skaya, and L. S. Éfros, Khim. Geterotsikl. Soedin., No. 6, 818 (1976).
M. J. Dewar, Molecular Orbital Theory of Organic Chemistry, McGraw-Hill (1969).
N. S. Loseva, L. E. Nivorozhkin, N. I. Borisenko, and V. I. Minkin, Khim. Geterotsikl. Soedin., No. 11, 1485 (1974).
V. I. Pantsyrnyi, M. A. Gal'bershtam, and N. A. Donskaya, Khim. Geterotsikl. Soedin., No. 5, 653 (1973).
M. B. Gordin and M. A. Gal'bershtam, Kinet. Katal., 12, 774 (1971).
E. Berman, R. E. Fox, and F. D. Thomson, J. Am. Chem. Soc., 81, 5605 (1959).
B. Ya. Simkin, V. I. Minkin, and V. A. Bren', Zh. Org. Khim., 13, 1910 (1977).
B. Tinland and C. Decoret, Gazz. Chim. Ital., 101, 792 (1971).
N. Tyutyulkov, S. Stoyanov, M. Taseva, and P. Schuster, J. Signal AM, 3, 435 (1975).
N. Mataga and K. Nishimoto, Z. Phys. Chem. (Frankfurt), 13, 140 (1957).
G. Arnold and G. Paal, Tetrahedron, 27, 1699 (1971).
V. I. Minkin, L. E. Nivorozhkin, N. S. Trofimova, Yu. V. Revinskii, M. I. Knyazhanskii, N. V. Volbushko, and B. Ya. Simkin, Zh. Org. Khim., 11, 828 (1975).
R. Exelby and R. Grinter, Chem. Rev., 65, 247 (1965).
É. R. Zakhs, L. A. Zvenigorodskaya, and L. S. Éfros, Khim. Geterotsikl. Soedin., No. 12, 1618 (1973).
Ya. N. Malkin, V. A. Kuz'min, G. G. Dyadyusha, A. N. Boguslavskaya, and F. A. Mikhailenko, Izv. Akad. Nauk SSSR, Ser. Khim., No. 3, 555 (1976).
A. I. Kiprianov, Usp. Khim., 40, 1283 (1971).
Ya. N. Malkin, V. A. Kuz'min, and F. A. Mikhailenko, Izv. Akad. Nauk SSSR, Ser. Khim., No. 1, 83 (1977).
R. S. Becker and J. Kolc, J. Phys. Chem., 72, 997 (1968).
V. I. Minkin, B. Ya. Simkin, L. P. Olekhnovich, and M. I. Knyazhanskii, Teor. Exp. Khim., 10, 668 (1974).
B. Ya. Simkin, V. I. Minkin, and V. I. Natanzon, Summaries of Papers Presented at the Sixth All-Union Conference on Quantum Chemistry [in Russian], Shtiintsa, Kishinev (1975), p. 157.
Author information
Authors and Affiliations
Additional information
See [1] for communication VIII.
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 9, pp. 1180–1192, September, 1978.
Rights and permissions
About this article
Cite this article
Simkin, B.Y., Minkin, V.I. & Nivorozhkin, L.E. Photochromic and thermochromic spirans. Chem Heterocycl Compd 14, 948–959 (1978). https://doi.org/10.1007/BF00509547
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00509547