Abstract
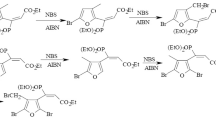
It is shown that the reactivities of cyclic formals in the case of bromination with dioxane dibromide increase in the order 1,3-dioxepane > 1,3-dioxalane ≫ 1,3-dioxane, which is explained not only by steric factors but also by the ease of cleavage of the C4-O3 bond of the dioxacyclane ring. The bromination of cyclic acetals takes place through prior enolization of the cyclic acetal with subsequent electrophilic addition of bromine to the double bond.
Similar content being viewed by others
Literature cited
D. L. Rakhmankulov, V. S. Martem'yanov, S. S. Zlot-skii, and Z. L. Ayupova, Zh. Prikl. Khim., 48, 1165 (1975).
D. L. Rakhmankulov, V. S. Martem'yanov, S. S. Zlot-skii, Z. L. Ayupova, and T. S. Artamonova, Zh. Obshch. Khim., 45, 2739 (1975).
Z. L. Ayupova, V. S. Martem'yanov, E. T. Denisov, U. B. Imashev, and D. L. Rakmankulov, Izv. Akad. Nauk SSSR, Ser. Khim., No. 8, 1770 (1978).
E. Coene and M. Anteunis, Bull. Soc. Chim. Belges, 79, 37 (1970).
W. E. Willy, G. Binsch, and E. L. Eliel, J. Am. Chem. Soc., 92, 5394 (1970).
D. F. Bocian and H. L. Strauss, J. Am. Chem. Soc., 99, 2866 (1977).
G. L. Kamalov, N. G. Luk'yanenko, Yu. Yu. Samitov, and A. V. Bogatskii, Zh. Org. Khim., 13, 1095 (1977).
P. Watts, J. Chem. Soc., B, No. 3, 543 (1968).
J. Kovac, J. Stefkova, and J. Jary, Coll. Czech. Chem. Commun., 30, 2793 (1965).
A. Marquet, M. Kagan, M. Dvolaitsky, L. Mamlok, C. Weidemann, and J. Jacques, Compt. Rend., 248, 984 (1959).
A. V. Bogatskii (Bogatsky), G. L. Kamalov, N. G. Luk'yanenko (Lukjanenko), S. A. Kotlyar (Kotljar), M. Bartok, J. Thsombosh, and Yu. Yu. Samitov, Acta Chim. Acad. Sci. Hung., 86, 173 (1975).
B. Kehl (editor), Laboratory Technique of Organic Chemistry [Russian translation], Mir, Moscow (1966), p. 594.
A. Gordon and R. Ford, Chemist's Companion: A Handbook of Practical Data Techniques and References, Wiley (1973).
J. D. Billimoria and N. F. Maclagan, J. Chem. Soc., No. 12, 3259 (1954).
V. N. Alekseev, Quantitative Analysis [in Russian], Khimiya, Moscow (1972), p. 395.
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 2, pp. 176–179, February, 1981.
Rights and permissions
About this article
Cite this article
Kotlyar, S.A., Kamalov, G.L., Savranskaya, R.L. et al. Kinetics and mechanism of bromination of cyclic acetals with 1,4-dioxane dibromide. Chem Heterocycl Compd 17, 120–123 (1981). https://doi.org/10.1007/BF00507239
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00507239