Abstract
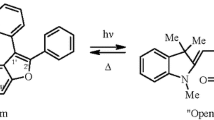
The mechanisms of the electrocyclic reactions of oxetene (I), 2H-pyran (II), 2-amino-2H-pyran (III), 2H-thiopyran (IV), and chromene (V) in the ground, first singlet, and tripletexcited stateswere studiedby means of the MINDO/3 method. The enthalpies of excitation are 125 (I, So), 32 (I, S1), 113 (I, T1), 188 (II, So), 109 (III, So), 150 (IV, So), and 239 kJ/mole (V, So). The number of isomers of the open form in So is determined by the nature of the substituent attached to the final carbon atom. It was observed that π donors stabilize both the transoid isomer and the cisoid isomer, which is twisted 20 ° with respect to the formally single β-γ carbon-carbon bond. Bulky substituents cause ∼105 ° rotation about the same bond.
Similar content being viewed by others
Literature cited
I. M. Andreeva, E. A. Bondarenko, N. V. Volbushko, M. I. Knyazhanskii, E. A. Medyantseva, A. V. Metelitsa, V. I. Minkin, and B. Ya. Simkin, Khim. Geterotsikl. Soedin., No. 8, 1035 (1980).
K. V. Veksler, É. R. Zakhs, V. M. Treiger, and L. S. Éfros, Khim. Geterotsikl. Soedin., No. 4, 447 (1971).
R. C. Bertelson, in: Photochromism, G. H. Brown, ed., Wiley Interscience, New York (1971), p. 45.
V. A. Barachevskii, G. I. Lashkov, and V. A. Tsekhomskii, Photochromism and Its Application [in Russian], Khimiya, Moscow (1977).
B. Ya. Simkin, V. I. Minkin, and L. E. Nivorozhkin, Khim. Geterotsikl. Soedin., No. 9, 1180 (1978).
B. Ya. Simkin and V. I. Minkin, Khim. Geterotsikl. Soedin., No. 2, 177 (1980).
B. Ya. Simkin, V. I. Minkin, and L. E. Nivorozhkin, Khim. Geterotsikl. Soedin., No. 1, 76 (1974).
B. Tinland and C. Decoret, J. Mol. Struct., 17, 414 (1973).
B. Tinland and C. Decoret, Gazz. Chim. Ital., 101, 792 (1971).
A. Samat, R. Guglielmetti, Y. Ferre, H. Pommier, and J. Metzger, J. Chim. Phys., 69, 1202 (1972).
R. Guglielmetti, J. Phot. Sci., 22, 77 (1974).
N. Tyutyulkov, S. Stoyanov, M. Taseva, and P. Shnster, J. Signal A. M., 3, 435 (1975).
H. Pommier, A. Samat, J. Metzger, M. Taseva, and R. Guglielmetti, J. Chim. Phys., 72, 589 (1975).
B. Tinland, R. Guglielmetti, and O. Chalvet, Tetrahedron, 29, 665 (1973).
V. I. Minkin, B. Ya. Simkin, L. E. Nivorozhkin, and B. S. Luk'yanov, Khim. Geterotsikl. Soedin., No. 1, 65 (1974).
V. R. Blok, Zh. Fiz. Khim., 52, 1683 (1978).
M. Barta, Collect. Czech. Commun., 43, 3339 (1978).
M. Barta, Collect. Czech. Commun., 43, 417 (1978).
R. C. Bingham, M. J. S. Dewar, and H. D. Lo, J. Am. Chem. Soc., 97, 1285 (1979).
K. Hau, R. J. Buenker, and S. D. Peyerimhoff, J. Am. Chem. Soc., 93, 5005 (1971).
R. J. Buenker, S. D. Peyerimhoff, and K. Hsu, J. Am. Chem. Soc., 93, 5005 (1971).
K. Hsu, R. J. Buenker, and S. D. Peyerimhoff, J. Am. Chem. Soc., 94, 5639 (1972).
A. T. Balaban, W. Schroth, and G. Fischer, Adv. Heterocycl. Chem., 10, 241 (1969).
E. N. Marvell, G. Caple, T. A. Gosink, and G. Zimmer, J. Am. Chem. Soc., 88, 619 (1966).
A. R. Katritzky, R. T. C. Brownlee, and G. Musumarra, Tetrahedron, 36, 1643 (1980).
R. S. Becker and J. Michl, J. Am. Chem. Soc., 88, 5931 (1966).
K. van der Meer and J. C. Mulder, Theor. Chim. Acta, 37, 159 (1975).
M. J. S. Dewar, Chem. Brit., 11, 97 (1975).
L. E. Friedrich and G. B. Schuster, J. Am. Chem. Soc., 93, 2602 (1971).
P. C. Martino and P. B. Shevlin, J. Am. Chem. Soc., 102, 5429 (1980).
J. M. Fiquera, P. B. Shevlin, and S. D. Worley, J. Am. Chem. Soc., 98, 3820 (1976).
M. J. S. Dewar and S. Kirschner, J. Am. Chem. Soc., 96, 6809 (1974).
R. Woodward and R. Hoffman, Conservation of Orbital Symmetry, Academic Press (1970).
A. Komornicki and J. M. McIver, Jr., J. Am. Chem. Soc., 96, 5798 (1974).
A. Devaquet, Top. Curr. Chem., 54, 1 (1975).
A. V. Zubkov and A. S. Kholmanskii, Dokl. Akad., Nauk SSSR, 229, 377 (1976).
A. V. Vannikov and A. Yu. Kryukov, Khim. Vys. Énerg., 12, 334 (1978).
A. Yu. Kryukov and A. V. Vannikov, Khim. Vys. Énerg., 12, 502 (1978).
V. A. Murin, V. F. Mandzhikov, and V. A. Barachevskii, Opt. Spektrosk., 37, 1174 (1974).
R. Osman, A. Zunger, and J. Shvo, Tetrahedron, 34, 2315 (1978).
R. Osman and J. Shvo, Tetrahedron, 34, 2320 (1978).
Author information
Authors and Affiliations
Additional information
See [1] for Communication 12.
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 8, pp. 1028–1036, August, 1982.
Rights and permissions
About this article
Cite this article
Simkin, B.Y., Makarov, S.P. & Minkin, V.I. Photo- and thermochromic spirans. 13. Calculation of the pathways of the thermal electrocyclic reactions of chromenes and their structural analogs by the MINDO/3 method. Chem Heterocycl Compd 18, 779–787 (1982). https://doi.org/10.1007/BF00506576
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00506576