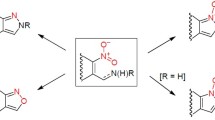
Abstract
The pathways and methods of transformation of isoxazoles to give various acyclic difunctional derivatives (α-cyano ketones, β-diketones, imino and amino ketones, β-amino alcohols, α,β-unsaturated ketones, etc.) by opening of the heteroring are examined in the review from the point of view of latent functionality. The prospects for the application of acyclic functional derivatives of isoxazoles and 2-isoxazolines — adducts of 1,3-dipolar cycloaddition of nitrile oxides to olefins and acetylenes — for regio- and stereospecific synthesis are discussed.
Similar content being viewed by others
Literature cited
A. I. Meyers, Heterocycles in Organic Synthesis, Wiley, New York-London (1974).
D. Lednicer, Adv. Org. Chem., 8, 179 (1972).
C. Kashima, Heterocycles, 12, 1343 (1979).
R. Barnes, in: Heterocyclic Compounds, Vol. 5, R. Elderfield, et. Wiley (1957).
A. Quilico, The Chemistry of Heterocyclic Compounds, Vol. 17, A. Weissberger, ed., Academic Press, New York (1963).
N. K. Kochetkov and S. D. Sokolov, in: Advances in Heterocyclic Chemistry, Vol. 2, Academic Press, New York (1963), p. 365.
C. Grundmann, Synthesis, No. 7, 344 (1970).
L. S. Crawley and W. J. Fanshawe, J. Heterocycl. Chem., 14, 531 (1977).
G. N. Barber and R. A. Olofson, J. Org. Chem., 43, 015 (1978).
A. A. Akhrem, F. A. Lakhvich, V. A. Khripach, and A. G. Pozdeev (Pozdeyev), Synthesis, No. 1, 43 (1978).
C. Grundmann and P. Grünanger, The Nitrile Oxides, Springer Verlag, Berlin (1971), p. 96.
T. Nishiwaki, Synthesis, No. 1, 20 (1975).
C. J. Subrahmanyam, Sci. Ind. Res., 33, 304 (1974).
S. D. Sokolov, Usp. Khim., 48, 533 (1979).
T. Kametani and H. Nemoto, Heterocycles, 10, 349 (1978).
T. Kametani and K. Fukumoto, Heterocycles, 10, 469 (1978).
V. Bertini, A. Munno, and P. Pino, Gazz. Chim. Ital., 97, 185 (1967).
A. Munno, V. Bertini, and F. Lucchesini, J. Chem. Soc., Perkin Trans. II, No. 9, 1121 (1977).
M. L. Casey, and D. S. Kemp, K. G. Paul, and D. D. Cox, J. Org. Chem., 38, 2294 (1973).
P. J. Vinick and H. W. Gschwend, Tetrahedron Lett., No. 44, 4221 (1978).
I. Hoppe and U. Schölkopf, Lieb. Ann., No. 2, 219 (1979).
D. S. Kemp, S. -W. Wang, R. C. Mollan, S. -L. Hsia, and P. N. Confalone, Tetrahedron, 30, 3677 (1974).
D. S. Kemp, S. -W. Wang, J. Rebek, R. C. Mollan, C. Banquer, and G. Subramanyam, Tetrahedron, 30 3955 (1974).
D. S. Kemp, S. J. Wrobel, S. -W. Wang, Z. Bernstein, and J. Rebek, Tetrahedron, 30, 3969 (1974).
G. Jones and J. R. Phipps, J. Chem. Soc., Perkin Trans. I, No. 11, 1241 (1976).
R. H. Good, G. Jones, and J. R. Phipps, Tetrahedron Lett., No. 7, 609 (1972).
R. H. Good, G. Jones, and R. Phipps, J. Chem. Soc., Perkin Trans. I, No. 19, 2441 (1972).
G. Jones and J. R. Phipps, J. Chem. Soc., Perkin Trans. I, No. 1, 158 (1974).
C. Kashima, N. Mukai, Y. Yamamoto, Y. Tsuda, and Y. Omote, Heterocycles, 7, 241 (1977).
N. K. Kochetkov and S. D. Sokolov, Zh. Obshch. Khim., 33, 1442 (1963).
M. I. Shevchuk, A. F. Tolochko, M. G. Bal'on, and M. V. Khalaturnik, Zh. Org. Khim., 14, 2003 (1978).
I. Adachi, K. Harada, R. Miyazaki, and H. Kano, Chem. Pharm. Bull, 22, 61 (1974).
Z. Jerzmanowsak and W. Basinski, Roczn. Chem., 51, 2283 (1977).
W. Basinski and Z. Jerzmanowska, Pol. J. Chem., 53, 229 (1979).
S. Auricchio, O. V. Pava, and E. Vera, Synthesis, No. 2, 116 (1979).
J. W. Scott and G. Saucy, J. Org. Chem., 37, 1652 (1972).
J. W. Scott, R. Borer, and G. Saucy, J. Org. Chem., 37, 1659 (1972).
C. Skotsch, I. Kohlmeyer, and E. Breitmaier, Synthesis, No. 6, 449 (1979).
G. Stork and J. E. McMurry, J. Am. Chem. Soc., 89, 5464 (1967).
P. Caramella, R. Metelli, and P. Grünanger, Tetrahedron, 27, 379 (1971).
A. A. Akhrem, V. A. Khripach, and F. A. Lakhvich, Khim. Geterotsikl. Soedin., No. 7, 901 (1974).
A. A. Akhrem, F. A. Lakhvich, V. A. Khripach, and A. G. Pozdeev, Dokl. Akad. Nauk Belorussk. SSR, 20, 1007 (1976).
A. A. Akhrem, F. A. Lakhvich, V. A. Khripach, and I. B. Klebanovich, Khim. Geterotsikl. Soedin., No. 3, 329 (1975).
A. A. Akhrem, F. A. Lakhvich, V. A. Khripach, and I. B. Klebanovich, Dokl. Akad. Nauk SSSR, 216, 1645 (1974).
A. A. Akhrem, F. A. Lakhvich, V. A. Khripach, and I. B. Klebanovich, Tetrahedron Lett., No. 44, 3983 (1976).
A. A. Akhrem, F. A. Lakhvich, V. A. Khripach, and I. B. Klebanovich, Khim. Geterotsikl. Soedin., No. 2, 230 (1979).
C. Kashima, J. Org. Chem., 40, 527 (1975).
G. Casnati, A. Quilico, A. Ricca, and P. Vita-Finzi, Chim. Ind., 47, 993 (1965).
S. Auricchio and A. Ricca, Gazz. Chim. Ital., 103, 37 (1973).
S. Auricchio, S. Morrocchi, and A. Ricca, Tetrahedron Lett., No. 33, 2793 (1974).
S. Auricchio, A. Ricca, and O. V. Pava, Chim. Ind., 58, 699 (1976).
T. Hiraoka, M. Yoshimoto, and Y. Kishida, Chem. Pharm. Bull., 20, 122 (1972).
I. Iijima, N. Taga, M. Miyazaki, and T. Tanaka, J. Chem. Soc., Perkin Trans. I, No. 12, 3190 (1979).
A. Barco, S. Benetti, and G. Pollini, Synth. Commun., 8, 219 (1978).
A. Barco, S. Benetti, G. P. Pollini, P. G. Baraldi, D. Simoni, and C. B. Vicentini, J. Org. Chem., 44, 1734 (1979).
H. A. Albrecht, J. F. Blount, F. M. Konzelmann, and J. T. Plati, J. Org. Chem., 44, 4191 (1979).
A. A. Akhrem, F. A. Lakhvich, and V. A. Khripach, Zh. Obshch. Khim., 45, 2572 (1975).
S. S. Mochalov, T. P. Surikova, and Yu. S. Shabarov, Khim. Geterotsikl. Soedin., No. 7, 886 (1976).
G. Dattolo, E. Aiello, S. Plescia, G. Cirrincione, and G. Daidone, J. Heterocycl. Chem., 14, 1021 (1977).
E. Aiello, G. Dattolo, G. Cirrincione, S. Plescia, and G. Daidone, J. Heterocycl. Chem., 15, 537 (1978).
A. Barco, S. Benetti, G. P. Pollini, P. G. Baraldi, M. Guarneri, and C. B. Vicentini, J. Org. Chem., 44, 105 (1979).
A. Camparini, F. Ponticelli, and P. Tedeschi, J. Heterocycl. Chem., 14, 435 (1977).
I. G. Markova, M. K. Polievktov, and S. D. Sokolov, Zh. Obshch. Khim., 46, 398 (1976).
R. C. Boruah, J. S. Sandhu, and G. Thyagarajan, J. Heterocycl. Chem., 16, 1087 (1979).
D. J. Andersen and A. Hassner, Synthesis, No. 8, 495 (1975).
K. Dietliker, P. Gilgen, H. Heimgarther, and H. Schmid, Helv. Chim. Acta, 59, 2074 (1976).
M. Nakagawa, T. Nakamura, and K. Tomita, Agric. Biol. Chem., 38, 2205 (1974).
J. P. Ferris and R. W. Trimmer, J. Org. Chem., 41, 14 (1976).
T. Sato and K. Saito, Chem. Commun., No. 19, 781 (1974).
T. Doppler, H. Schmid, and H. -J. Hansen, Helv. Chim. Acta, 62, 314 (1979).
E. Giovannini and B. F. S. E. Sousa, Helv. Chim. Acta, 62, 185 (1979).
E. Giovannini and B. F. S. E. Sousa, Helv. Chim. Acta, 62 198 (1979).
T. Doppler, H. Schmid, and H. -J. Hansen, Helv. Chim. Acta, 62, 271 (1979).
T. Doppler, H. Schmid, and H. -J. Hansen, Helv. Chim. Acta, 62, 304 (1979).
N. F. Haley, J. Org. Chem., 42, 3929 (1977).
T. N. Mitchell and B. Kleine, Tetrahedron Lett., No. 25, 2173 (1976).
R. Huisgen and M. Christl, Chem. Ber., 106, 3291 (1973).
R. Huisgen and M. Christl, Angew. Chem., 79, 471 (1967).
G. W. Moersch, E. L. Wittle, and W. A. Newklis, J. Org. Chem., 30, 1272 (1965).
G. Gerali, G. Sportoletti, G. Parini, and A. Ius, Farm. Sci. Ed., 24, 112 (1969).
G. Gerali, G. Parini, G. Sportoletti, and A. Ius, Farm. Sci. Ed., 24, 231 (1969).
G. Gerali, G. Parini, A. Ius, and G. Sportoletti, Farm. Sci. Ed., 24, 299 (1969).
V. Jäger and H. Grund, Angew. Chem., 88, 27 (1976).
H. Grund and V. Jäger, Lieb. Ann., No. 1, 80 (1980).
G. Bianchi, R. Gandolfi, and P. Grünanger, J. Heterocycl. Chem., 5, 49 (1968).
G. Bianchi, A. Gamba-Invernizzi, and R. J. Gandolfi, J. Chem. Soc., Perkin Trans I, No. 15, 1757 (1974).
R. G. Shotter, D. Sesardic, and P. H. Wrigt, Tetrahedron, 31, 3069 (1975).
H. Grund and V. Jäger, J. Chem. Res. (Synop.), No. 2, 54 (1979); Chem. Abstr., 91, 91541 (1979).
W. Stühner and W. Heinrich, Chem. Ber., 84, 224 (1951).
G. Drefahl and H. H. Hörhold, Chem. Ber., 97, 159 (1964).
T. Kusumi, H. Kakisawa, S. Suzuki, K. Harada, and C. Kashima, Bull. Chem. Soc. Jpn., 51, 1261 (1978).
K. Torsell and O. Zeuthen, Acta Chem. Scand., B32, 118 (1978).
A. A. Akhrem, F. A. Lakhvich, V. A. Khripach, and I. I. Petrusevich, Khim. Geterotsikl. Soedin., No. 7, 891 (1976).
A. A. Akhrem, F. A. Lakhvich, V. A. Khripach, I. B. Klebanovich, and A. G. Pozdeev, Khim. Geterotsikl. Soedin., No. 5, 625 (1976).
A. A. Akhrem, F. A. Lakhvich, V. A. Khripach, and I. B. Klebanovich, Dokl. Akad. Nauk SSSR, 244, 615 (1979).
D. N. Kursanov, Z. N. Parnes, M. I. Kalinkin, and N. M. Loim, Ionic Hydrogenation [in Russian], Khimiya, Moscow (1979), p. 167.
A. A. Akhrem, F. A. Lakhvich, V. A. Khripach, and I. B. Klebanovich, USSR Inventor's Certificate No. 607832 (1978); Byull. Izobret., No. 19, 59 (1978).
G. W. Perold and F. V. K. Reiche, J. Am. Chem. Soc., 79, 465 (1957).
N. Barbulescu and A. Quilico, Gazz. Chim. Ital., 92, 326 (1961).
P. Grünanger and M. R. Langella, Gazz. Chim. Ital., 91, 1112 (1961).
N. Barbulescu, P. Grünanger, M. R. Langella, and A. Quilico, Tetrahedron Lett., No. 2, 89 (1961).
R. Paul and S. Tchelitcheff, Bull. Soc. Chim. Fr., Nos. 11–12, 2215 (1962).
M. V. Kashutina, S. L. Ioffe, and V. A. Tartakovskii, Dokl. Akad. Nauk SSSR, 218, 109 (1974).
V. Jäger, V. Buss, and W. Schwab, Tetrahedron Lett., No. 34, 3133 (1978).
V. Jäger and V. Buss, Lieb. Ann., No. 1, 101 (1980).
V. Jäger, V. Buss, and W. Schwab, Lieb. Ann., No. 1, 122 (1980).
K. F. Burri, R. A. Cardone, Wen Yean Chen, and P. Rosen, J. Am. Chem. Soc., 100, 7069 (1978).
T. Wieland, Naturwissenschaften, 59, 225 (1972).
A. Gieren, P. Narayanan, W. Hoppe, M. Hasan, K. Michl, T. Wieland, H. O. Smith, G. Jung, and E. Breitmaier, Lieb. Ann., No. 10, 1561 (1974).
H. V. Secor and E. B. Sanders, J. Org. Chem., 43, 2539 (1978).
K. Kotera, Y. Takano, A. Matsuura, and K. Kitahonoki, Tetrahedron Lett., No. 55, 5759 (1968).
K. Kotera, Y. Takano, A. Matsuura, and K. Kitahonoki, Tetrahedron, 26, 539 (1970).
K. Kitahonoki, K. Kotera, Y. Matsukava, S. Miyazaki, T. Okada, H. Takahashi, and Y. Takano, Tetrahedron Lett., No. 16, 1059 (1965).
G. S. King, P. D. Magnus, and H. S. Rzepa, J. Chem. Soc., Perkin Trans. I, No. 3, 437 (1972).
V. Jäger, H. Grund, and W. Schwab, Angew. Chern., Int. Ed., 18, 78 (1979).
G. W. Perold and F. V. K. Reiche, J. South Afr. Chem. Inst., 10, 5 (1957); Chem. Abstr., 52, 1145 (1958).
G. S. Alcontres and G. L. Vecchio, Gazz. Chim. Ital., 90, 1239 (1960).
Y. Ito and T. Matsuura, Tetrahedron, 31, 1373 (1975).
H. Saiki, T. Miyashi, and T. Mukai, Tetrahedron Lett., No. 52, 4619 (1977).
O. Sechimoto, T. Kumagai, K. Shimizu, and T. Mukai, Chem. Lett., No. 10, 1195 (1977); Curr. Abstr., 68, 265237 (1978).
H. Kaufmann and J. Kalvoda, Chem. Commun., No. 6, 210 (1976).
G. Bianchi and P. Grünanger, Tetrahedron, 21, 817 (1965).
A. Barco, S. Benetti, and G. Pollini, Synthesis, No. 12, 837 (1977).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 9, pp. 1155–1173, September, 1981.
Rights and permissions
About this article
Cite this article
Akhrem, A.A., Lakhvich, F.A. & Khripach, V.A. Isoxazole derivatives in the synthesis of bifunctional compounds by cleavage of the heteroring (review). Chem Heterocycl Compd 17, 853–868 (1981). https://doi.org/10.1007/BF00505584
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00505584