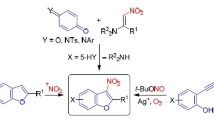
Abstract
When benzofuran is alkylated with tert-butyl chloride in the vapor phase (190–260‡C) in a flow system, the ratio of the 2- and 3-tert-butylbenzofurans formed varies from 2∶1 to 20∶1, depending on the acidity of the catalyst used (ZnCl2/Al2O3), because of conversion of the 3-isomer to the 2-isomer.
Similar content being viewed by others
Literature cited
é. A. Karakhanov, G. V. Drovyannikova, L. A. Kiseleva, and E. A. Viktorova, Khim. Geterotsikl. Soedin., 1020 (1971).
é. A. Karakhanov, G. V. Drovyannikova, and E. A. Viktorova, Khim. Geterotsikl. Soedin., 156 (1971).
A. N. Kost, V. A. Budylin, E. D. Matveeva, and D. O. Sterligov, Zh. Organ. Khim., 6, 1503 (1970).
Author information
Authors and Affiliations
Additional information
See [1] for communication III.
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 2, pp. 172–175, February, 1974.
Rights and permissions
About this article
Cite this article
Karakhanov, é.A., Drovyannikova, G.V. & Viktorova, E.A. Catalytic transformations of benzofuran. Chem Heterocycl Compd 10, 151–153 (1974). https://doi.org/10.1007/BF00487768
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00487768