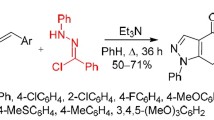
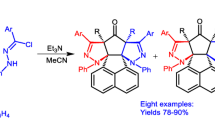
Abstract
The interaction of substituted trans-1,1-dicyano-4-phenyl-1,3-butadienes with pyridinium ylides proceeds through the path of [π4s+π2s]-dipolar cycloaddition, highly regioselective and stereoselective, with the formation of substituted 1-(2,2-dicyanovinyl)-2-phenyl-6-cyano-1,2-trans-2,3-trans-1,9-trans-1,2,3,9-tetrahydroindolizines. The stereoselectivity of these reactions is due to endo-addition of molecules of trans-1,3-butadienes to the anti form of the pyridinium ylides; it is confirmed by correlation analysis of the data from double PMR, mathematical calculations of torsion angles, and x-ray structural studies of the substituted tetrahydroindolizines.
Similar content being viewed by others
Literature Cited
A. M. Shestopalov, Yu. A. Sharanin, V. P. Litvinov, and O. M. Nefedov, Zh. Org. Khim., 25, 1111 (1989).
A. M. Shestopalov, Yu. A. Sharanin, L. A. Rodinovskaya, V. Yu. Mortikov, V. P. Litvinov, and V. K. Promonenkov, in: Summaries of Papers from First All-Union Conference on the Chemistry and Technology of Pyridine-Containing Pesticides, Chernogolovka (1988), p. 122.
A. M. Shestopalov, Yu. A. Sharanin, V. N. Nesterov, L. A. Rodinovskaya, V. E. Shklover, Yu. T. Struchkov, and V. P. Litvinov, Khim. Geterotsikl. Soedin., No. 9, 1248 (1991).
J. Zugravescu and M. N. Petrovanu, Ylid Chemistry, McGraw-Hill, New York (1976).
O. Tsuge, S. Kanemasa, and S. Takenaka, Bull. Chem. Soc. Jpn., 58, 3137 (1985).
O. Tsuge, S. Kanemasa, and K. Samato, Bull. Chem. Soc. Jpn., 61, 2513 (1988).
T. L. Gilchrist and R. C. Storr, Organic Reactions and Orbital Symmetry, Cambridge University Press (1972).
H. Guenther, NMR Spectroscopy: An Introduction, Wiley, New York (1980).
N. S. Zefirov and V. A. Palyulin, Dokl. Akad. Nauk SSSR, 252, 111 (1980).
D. Cremer and J. A. Pople, J. Am. Chem. Soc., 97, 1354 (1975).
F. H. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen, and R. Taylor, J. Chem. Soc., Perkin Trans. 2, No. 1, 1 (1987).
L. C. Groenen, W. Verboom, W. H. N. Nyhuis, D. N. Reinhoudt, G. J. van Hummel, and D. Feil, Tetrahedron, 44, 4637 (1988).
A. Bondi, J. Phys. Chem., 70, 3006 (1966).
R. G. Gerr, A. I. Yanovskii, and Yu. T. Struchkov, Kristallografiya, 28, 1029 (1983).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 10, pp. 1354–1360, October, 1991.
Rights and permissions
About this article
Cite this article
Shestopalov, A.M., Sharanin, Y.A., Nesterov, V.N. et al. Stereoselective [π4s+π2s]-dipolar cycloaddition of pyridinium ylides to nitriles of the trans-1,3-butadiene series. Crystal and molecular structure of 3-benzoyl-1-(2,2-dicyano-1-cyclopropylvinyl)-2-phenyl-6-cyano-1,2-trans-2,3-trans-1,9-trans-1,2,3,9-tetrahydroindolizine. Chem Heterocycl Compd 27, 1084–1090 (1991). https://doi.org/10.1007/BF00486803
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00486803