Abstract
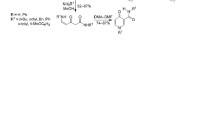
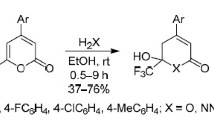
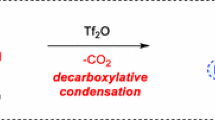
Saponification of the appropriate 3-carbethoxy-α-pyrones gives α-pyrone-3-carboxylic acid and its homologs, further converted via their acid chlorides to the substituted amides of the acids.
Similar content being viewed by others
References
N. K. Kochetkov and L. I. Kudryashov, ZhOKh, 27, 248, 1957.
N. K. Kochetkov and L. I. Kudryashov, ZhOKh, 28, 1511, 1958.
Z. I. Shramova, T. V. Protopopova, and A. P. Skoldinov, ZhOKh, 34, 3511, 1964.
Z. I. Shramova, V. G. Vinokurov, and A. P. Skoldinov, ZhOrKh, 2, 346, 1966.
H. Pehmann, Ann., 264, 261, 1891.
F. Goss, C. Ingold, and J. Thorpe, J. Chem. Soc., 123, 3342, 1923.
S. Ruhemann, J. Chem. Soc., 75, 245, 1899.
R. Wiley, N. Smith, and J. Baner, J. Am. Chem. Soc., 75, 244, 1953.
N. K. Kochetkov, L. I. Kudryashov, and T. M. Senchenkova, ZhOKh, 28, 3020, 1958.
T. Windholz, L. Peterson, and G. Kent, J. Org. Chem., 28, 1443, 1963.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shramova, Z.I., Skoldinov, A.P. Some derivatives of α-pyrone-3-carboxylic acid and its homologs. Chem Heterocycl Compd 3, 475–477 (1967). https://doi.org/10.1007/BF00481574
Issue Date:
DOI: https://doi.org/10.1007/BF00481574