Abstract
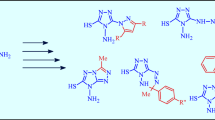
Treatment of mono- and diisocyanates with 3-methyl-4-alkyl(aryl)pyrazol-5-ones by heating in absolute dioxane gives the corresponding 1-carbamoylpyrazol-5-ones. Using PMR spectroscopy it has been shown that they exist in the 5-hydroxypyrazole form in DMSO, dimethylacetamide, chloroform, or acetone solutions.
Similar content being viewed by others
Literature Cited
S. Petersen, Ann. Chemie, 562, 205 (1949).
German Patent No. 3,114,833, Abstract of Applications, No. 43, Part 1 (1982).
T. L. Jacobs, in: Heterocyclic Compounds, R. Elderfield (ed.) [Russian translation], Vol. 5, Mir, Moscow (1961), p. 84.
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 8, pp. 1058–1060, August, 1990.
Rights and permissions
About this article
Cite this article
Vostokov, I.A., Vasyaeva, L.V., Maricheva, G.A. et al. Reaction of isocyanates with pyrazol-5-ones. Chem Heterocycl Compd 26, 884–886 (1990). https://doi.org/10.1007/BF00480862
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00480862