Abstract
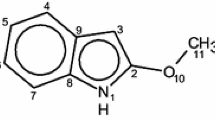
Isomers of 2-methyl- and 1,2-dimethyl-cis-decahydro-5-quinolinol with a syn orientation of the hydroxy and amino groups and different orientations of the methyl group relative to the methylene group at C(8)H2 were subjected to conformational analysis. In the case of a cis orientation of the methyl and methylene groups the equilibrium is shifted completely to favor the conformation with an intramolecular hydrogen bond, whereas in the case of their trans orientation the mole fraction of this conformation amounts to 21–24% for the secondary amino alcohol and 18–21% for the tertiary amino alcohol. The energies of the hydrogen bonds were determined from the intensities of the absorption bands of the free and associated hydroxy groups in the IR spectra: for the secondary hydroxy amine, according to the band of the free hydroxy group, δG0 OH/N is −0.8 kcal/mole, whereas, according to the band of the associated hydroxy group, it is −0.9 kcal/mole; the values for the tertiary hydroxy amine are, respectively, −0.7 and −0.8 kcal/mole.
Similar content being viewed by others
Literature Cited
G. S. Litvinenko and L. A. Voronenko, Khim. Geterotsikl. Soedin., No. 2, 238 (1987).
N. Yu. Kuz'mina, Zh. I. Isin, D. V. Sokolov, and G. S. Litvinenko, Izv. Akad. Nauk Kaz. SSR, Ser. Khim., No. 1, 55 (1981).
D. V. Sokolov, N. Yu. Kuz'mina, Zh. I. Isin, and G. S. Litvinenko, Izv. Akad. Nauk Kaz. SSR, Ser. Khim., No. 4, 59 (1981).
G. S. Litvinenko, N. Yu. Kuz'mina, and D. V. Sokolov, Khim. Geterotsikl. Soedin., No. 3, 382 (1984).
A. A. Espenbetov, Yu. T. Struchkov, N. Yu. Kuz'mina, and G. S. Litvinenko, Izv. Akad. Nauk Kaz. SSR, Ser. Khim., No. 5, 59 (1982).
H. S. Aaron and C. P. Ferguson, J. Am. Chem. Soc., 98, 7013 (1976).
H. A. Aaron and C. P. Ferguson, Tetrahedron, 30, 803 (1974).
H. S. Aaron and C. P. Ferguson, J. Org. Chem., 40, 3214 (1975).
H. S. Aaron, C. P. Ferguson, and C. P. Rader, J. Am. Chem. Soc., 89, 1431 (1967).
E. Eliel, N. Allinger, S. Angyal, and G. Morrison (eds.), Conformational Analysis, Wiley-Interscience, New York (1965).
H. Booth and J. M. Bailey, J. Chem. Soc., Perkin Trans., No. 2, 510 (1979).
B. J. Brignell, K. Brown, and A. R. Katritzky, J. Chem. Soc., B, No. 12, 1462 (1968).
H. Booth and D. V. Griffiths, Chem. Commun., No. 18, 666 (1973).
Author information
Authors and Affiliations
Additional information
See [1] for Communication 61.
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 11, pp. 1514–1519, November, 1987.
Rights and permissions
About this article
Cite this article
Litvinenko, G.S., Kuz'mina, N.Y. Stereochemistry of nitrogen heterocycles. 62. Conformational analysis of isomers of 2-methyl-cis-decahydro-5-quinolinol. Chem Heterocycl Compd 23, 1211–1215 (1987). https://doi.org/10.1007/BF00479371
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00479371