Abstract
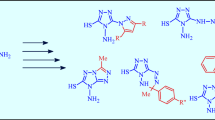
Reduction of 2-benzoylbenzoxazole oxime gives 2-(α-aminobenzyl) benzoxazole, converted to the formyl or acetyl derivative by treatment with, respectively, ethyl formate or acetic anhydride. Thiourea derivatives are obtained by treating 2-(α-aminobenzyl) benzoxazole with arylisothiocyanates. Heating the above formyl or acetyl derivative with phosphorus oxychloride converts them to 3-phenyl- and 3-phenyl-1-methylimidazo [5, 1-b]-benzoxazole, which are representative members of a new tricyclic system. It did not prove possible to cyclize 1-[α-(benzoxazolyl-2) benzyl]-3-phenylthiourea.
Similar content being viewed by others
References
S. Skraup, Ann. Chim., 419, 79, 1919.
S. Skraup and M. Moser, Ber., 55, 1095, 1922.
V. V. Avidon and M. N. Shchukina, KhGS [Chemistry of Heterocyclic Coumpounds], 64, 1965.
V. V. Avidon and M. N. Shchukina, KhGS [Chemistry of Heterocyclic Compounds], 349, 1965.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sycheva, T.P., Kiseleva, I.D. & Shchukina, M.N. Synthesis of 3-phenyl- and 3-phenyl-1-methylimidazo [5, 1-b] benzoxazoles. Chem Heterocycl Compd 2, 529–531 (1966). https://doi.org/10.1007/BF00477511
Issue Date:
DOI: https://doi.org/10.1007/BF00477511