Abstract
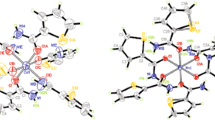
X-Ray diffraction examination and MNDO calculations have shown that 1,3,2-benzodithiazolium chloride (I) is ionic, with a planar heteroaromatic 10π-electron cation. The π-MO of the cation (I) is isolobal with the p-MO of benzo-2,1,3-thiadiazole. In the cation of (I), as in the latter compound, the p-AO of nitrogen and sulfur contribute for the most part to π-MO of differing symmetry (b1 and a2, respectively). This has the consequence that although both nitrogen and sulfur participate in the formation of a single π-system in the thiazolium cation of (I), there is virtually no π-bonding between them. Generally speaking, the π-MO of the (I) cation shows a tendency to localization on separate molecular fragments. The charge on the cation is localized at the SNS group, and the five-membered ring is strongly polarized. These features all reduce the heteroaromaticity of the system.
Similar content being viewed by others
Literature Cited
G. Wolmershauser, M. Schnauber, and T. Wilhelm, J. Chem. Soc., Chem. Commun., 573 (1984).
G. Wolmershauser, M. Schnauber, and T. Wilhelm, Mol. Cryst. Liq. Cryst., 120, 323 (1985).
G. Wolmershauser, M. Schnauber, T. Wilhelm, and L. H. Sutcliff, Synth. Met., 14, 239 (1986).
K. A. Williams, M. J. Nowak, E. Dormann, and F. Wudi, Synth. Met., 14, 233 (1986).
S. W. Schneller, Int. J. Sulfur Chem., 8, 579 (1976).
R. Mayer, Phosphorus and Sulfur, 23, 277 (1985).
A. R. Katritzky and C. W. Rees (eds.), Comprehensive Heterocyclic Chemistry, Pergamon Press, Oxford (1984), Vol. 6, p. 898.
G. K. MacLean, J. Passmore, M. J. Schriver, P. S. White, D. Bethell, R. S. Pilkington, and L. H. Sutcliff, J. Chem. Soc., Chem. Commun., 807 (1983).
A. V. Zibarev, A. V. Rogoza, S. N. Konchenko, M. A. Fedotov, and G. G. Furin, Zh. Obshc. Khim.
K. L. Park, D. S. Stephenson, A. M. Gryff-Keller, and P. Szczecinski, Magn. Reson. Chem., 24, 831 (1986).
A. F. Pozharskii, Khim. Geterotsikl. Soedin., No. 7, 867 (1985).
L. V. Vilkov, V. S. Mastryukov, and N. I. Sadova, Determination of the Geometric Structure of Free Molecules [in Russian], Khimiya, Leningrad (1978), p. 180.
J. Leitch, S. C. Nyburg, D. A. Armitage, and M. J. Clark, J. Cryst. Mol. Struct., 3, 337 (1973).
F. P. Olsen and J. C. Barrick, Inorg. Chem., 12, 1353 (1973).
Yu. V. Zefirov and P. M. Zorkii, Zh. Strukt. Khim., 17, 994 (1976).
A. V. Zibarev, O. M. Fugaeva, A. A. Miller, S. N. Konchenko, I. K. Korobeinicheva, and G. G. Furin, Khim. Geterotsikl. Soedin., No. 8, 1124 (1990).
G. N. Dolenko, Structure of Matter and the Properties of Molecules [in Russian], Izd. DVGU, Vladivostok (1987), p. 225.
A. A. Bliznyuk, A. A. Voityuk, and L. N. Shchegoleva, Informational Materials of the Specialized File of Quantum Chemical Programs of the Siberian Section, Academy of Sciences of the USSR, Novosibirsk, IKhKiG, Siberian Section, Academy of Sciences of the USSR (1989), No. 3, SFKP-78.
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 12, pp. 1683–1688, December, 1990.
Rights and permissions
About this article
Cite this article
Zibarev, A.V., Bagryanskaya, I.Y., Latilov, Y.V. et al. Crystal, molecular, and π-electronic structure of 1,3,2-benzodithiazolium chloride. Chem Heterocycl Compd 26, 1399–1404 (1990). https://doi.org/10.1007/BF00473972
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00473972