Abstract
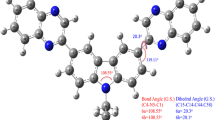
When carbazole is heated with formaldehyde and acetone (or with methyl vinyl ketone) in the presence of mineral acid, 3-methylpyrido[3,2-j,k]carbazolium salts form. These react with p-dimethylaminobenzaldehyde, triethyl orthoformate, Michler's ketone, 1-ethylquinolinium iodide, or 1,3,3-trimethyl-2-formylmethyleneindolinium iodide to give the respective cyanine dyes. Deviations of the unsymmetrical dyes and Hückel molecular orbital calculations show that the Brooker basicity of the pyrido[3,2,1-j,k]carbazolium series is lower than that of the 1-phenylquinolinium series.
Similar content being viewed by others
Literature Cited
G. T. Pilyugin and B. M. Gutsulyak, USSR Inventor's Certificate No. 103319; Byull. Izobret., No. 19, 12 (1956).
G. T. Pilyugin and B. M. Gutsulyak, Zh. Obshch. Khim., 29, 3076 (1969).
D. W. Heseltine and L. G. S. Brooker, US Patent 2578304; Chem. Abstr., 46, 8680 (1952).
D. W. Heseltine and L. G. S. Brooker, US Patent 2636035; Chem. Abstr., 48, 2785 (1954).
L. G. S. Brooker and D. W. Heseltine, British Patent 713252; Chem. Abstr., 49, 2913 (1955).
V. F. Traven, V. A. Plakhov, and B. I. Stepanov, Khim. Geterotsikl. Soedin., No. 4, 756 (1967).
I. N. Chernyuk, V. E. Pridan, V. A. Bazhutin, and M. Yu. Kornilov, Zh. Obshch. Khim., 44, 1584 (1974).
K. D. Bartle, D. W. Jones, and J. E. Pearson, J. Mol. Spectrosc., 24, 330 (1967).
G. M. Oksengendler and A. I. Kiprianov, Ukr. Khim. Zh., 16, 383 (1950).
G. T. Pilyugin, B. M. Gutsulyak, and Ya. O. Gorichok, Zh. Obshch. Khim., 34, 2412 (1964).
A. I. Kiprianov and G. T. Pilyugin, Uch. Zap. Kar'k. Gos. Un-ta, 10, 104 (1937).
L. G. S. Brooker, Rev. Modern Physics, 14, 275 (1942).
L. G. S. Brooker and R. H. Sprague, J. Am. Chem. Soc., 63, 3203 (1941).
W. König, Berichte, 57, 685 (1924).
G. T. Pilyugin and B. M. Gutsulyak, Zh. Obshch. Khim., 30, 1299 (1960).
G. G. Dyadyusha and A. D. Kachkovskii, Ukr. Khim. Zh., 44, 948 (1978).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 8, pp. 1059–1062, August, 1987.
Rights and permissions
About this article
Cite this article
Gutsulyak, B.M., Turov, A.V., Petrovskii, R.S. et al. Synthesis of 3-methylpyrido[3,2,1-j,k]carbazolium salts and derived cyanine dyes. Chem Heterocycl Compd 23, 846–849 (1987). https://doi.org/10.1007/BF00473454
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00473454