Abstract
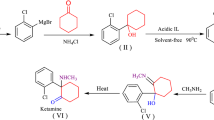
1, 2, 5-Trimethyl-4-phenylethynylpiperidol-4 and 1 2, 5-trirnethyl-4-ß-phenylethynylpiperidol-4 are prepared by various methods. Their interconversion is studied, and individual stereoisomeric forms of these piperidols isolated. 1, 2, 5-Trimethyl-4-phenylethynylpiperidol-4 is hydrated, and the resultant ω -(1,′ 2′, 5′-trimethyldidehydropiperidyl-4′) acetophenone used to effect synthesis of a number of secondary and tertiary carbinols. 2, 5-Dimethyl-4-ß-phenylethylpyridine is prepared from 1, 2, 5-trimethyl-4-ß-phenylethylpiperidol-4.
Similar content being viewed by others
References
I. N. Nazarov and V. A. Rudenko, Izv. AN SSSR, OKhN, 610, 1948.
I. N. Nazarov, N. S. Prostakov, Zh. A. Krasnaya, and N. N. Mikheeva, ZhOKh, 26, 2820, 1956.
I. N. Nazarov, N. S. Prostakov, and N. I. Shvetsov, ZhOKh, 26, 2798, 1956.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Prostakov, N.S., Kurichev, V.A. 1, 2, 5-Trimethyl-4-phenylethynylpiperidol-4 and its reactions. Chem Heterocycl Compd 1, 576–581 (1966). https://doi.org/10.1007/BF00472693
Issue Date:
DOI: https://doi.org/10.1007/BF00472693