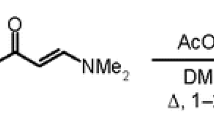
Abstract
The recyclization of pyrrolo[1,2-a]pyrazinium salts under the influence of a base leads to 8-aminoindolizine derivatives and is a new method for the synthesis of indolizines, as well as the first example of the Kost—Sagitullin rearrangement in the pyrazine series.
Similar content being viewed by others
Literature Cited
V. I. Terenin, E. V. Kabanova, E. S. Feoktistova, and Yu. G. Bundel', Khim. Geterotsikl. Soedin., No. 3, 424 (1989).
A. N. Kost, R. S. Sagitullin, and S. P. Gromov, Heterocycles. Special Issue, 7, 997 (1977).
A. M. Likhosherstov, V. P. Peresada, V. G. Vinokurov, and A. P. Skoldinov, Zh. Org. Khim., 22, 2610 (1986).
R. Rydzkowsky, D. Blondeau, and H. Sliva, Tetrahedron Lett., 26, 2571 (1985).
Author information
Authors and Affiliations
Additional information
See [1] for our preliminary communication.
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 6, pp. 763–766, June, 1991.
Rights and permissions
About this article
Cite this article
Terenin, V.I., Kabanova, E.V. & Bundel', Y.G. Conversion of pyrrolo[1,2-α]pyrazinium salts to 8-aminoindolizines. Chem Heterocycl Compd 27, 597–600 (1991). https://doi.org/10.1007/BF00472505
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00472505