Abstract
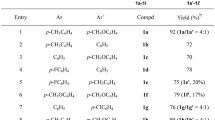
It is known that 1-arylthiosemicarbazides are cyclized to 2-aminobenzothiazoles in acidic media. This reaction was extended to 1,3- and 1,4-disubstituted thiosemicarbazides. It is shown that the rearrangement proceeds through a step involving the formation of o-aminophenylisothioureas. The reaction conditions and substituents in the benzene ring or attached to the nitrogen atoms of the thiosemicarbazides do not have a substantial effect on the ratio of the resulting 2-amino- and 2-phenyl(methyl)aminobenzothiazoles (1∶1). In the case of m-chlorophenylthiosemicarbazide, 5- and 7-chloro-2-aminobenzothiazoles are obtained in equimolar amounts, whereas the 7 isomer (5∶2) is the major product from m-tolylthiosemicarbazide.
Similar content being viewed by others
Literature cited
E. Fischer and E. Besthorn, Liebigs Ann., 212, 316 (1882).
C. D. Harris and E. Loewenstein, Ber., 27, 861 (1894).
A. Hugershoff, Ber., 36, 3134 (1903).
A. N. Kost, G. A. Golubeva, and L. A. Sviridova, Khim. Geterotsikl. Soedin., No. 4, 495 (1973).
H. Clusius and H. Weisser, Helv. Chim. Acta, 35, 400 (1952).
F. Kurzer and P. M. Sanderson, J. Chem. Soc., 230 (1962).
I. I. Grandberg, Izv. Timiryazev, Sel'skokhoz. Akad., No. 5, 186 (1972).
A. N. Kost, N. Yu. Lebedenko, and L. A. Sviridova, Zh. Org. Khim., 12, 2453 (1976).
A. R. Katritzky and Y. Takeuchi, Org. Magn. Res., 2, 569 (1970).
J. Paris, J. Couquelet, and P. Tronche, Compt. Rend., C, 272, 679 (1971).
E. E. Reid, Organic Chemistry of Bivalent Sulfur, Vol. 1, New York (1958), p. 32.
E. E. Reid, Organic Chemistry of Bivalent Sulfur, Vol. 3, New York (1960), p. 362.
G. W. Ewans and B. Milligan, Austr. J. Chem., 20, 1779 (1967).
P. B. Terent'ev, R. A. Khmel'nitskii, I. S. Khromov, A. N. Kost, I. P. Gloriozov, and M. Islam, Zh. Org. Khim., 6, 606 (1970).
A. A. Polyakova and R. A. Khmel'nitskii, Mass Spectrometry in Organic Chemistry [in Russian], Khimiya, Leningrad (1972), p. 213.
M. Marx and C. Djerassi, J. Am. Chem. Soc., 90, 678 (1968).
P. A. Sharbatyan, N. Yu. Lebedenko, and A. N. Kost, Zh. Org. Khim., 13 (1977).
I. G. Farben, A. G. French Patent No. 688867 (1930); Chem. Abstr., 25, 968 (1931).
R. F. Hunter, J. Chem. Soc., 1385 (1926).
P. T. Paul and L. B. Tewksbury, US Patent No. 2435508 (1948); Chem. Abstr., 42, 5050 (1948).
G. M. Dyson and T. Harrington, J. Chem. Soc., 191 (1940).
R. F. Hunter and M. A. Wali, J. Chem. Soc., 1513 (1937).
R. Riemschneider and S. Georgi, Monatsh. Chem., 91, 630 (1960).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 4, pp. 467–475, April, 1978.
Rights and permissions
About this article
Cite this article
Kost, A.N., Lebedenko, N.Y., Sviridova, L.A. et al. Cyclization of 1-arylthiosemicarbazides to benzothiazoles. Chem Heterocycl Compd 14, 380–388 (1978). https://doi.org/10.1007/BF00472148
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00472148