Abstract
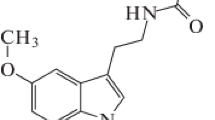
On the basis of data from the IR spectra of 2-acetylindoles and their pyrrole-ring-substituted derivatives it is concluded that these substances exist in CCl4 solution primarily in the s-trans conformation.
Similar content being viewed by others
Literature cited
A. Marinangelli, Ann. Chim. (Roma), 44, 219 (1954).
M. L. Martin, J.-C. Rose, and G. J. Martin, Tetrahedron Lett., 3407 (1970).
H. Fritzsche, Ber. Bunsenges. Phys. Chem., 68, 459 (1964).
T. Gramstad, Spectrochim. Acta, 19, 497 (1963).
L. J. Bellamy, Infrared Spectra of Complex Molecules, Methuen (1968).
R. N. Jones, W. F. Forbes, and W. A. Mueller, Can. J. Chem., 35, 504 (1957).
V. A. Budylin, A. N. Kost, E. D. Matveeva, and V. I. Minkin, Khim. Geterotsikl. Soedin., 68 (1972).
H. Laato and R. Isalato, Acta Chem. Scand., 21 2119 (1967).
I. M. Ginzburg and B. P. Tarasov, Zh. Obshch. Khim., 42, 2740 (1972).
L. Joris and P. R. Schleyer, J. Amer. Chem. Soc., 90, 4599 (1968).
A. N. Kost, V. A. Budylin, E. D. Matveeva, V. I. Gorbunov, S. M. Gorbunova, and T. G. Kharlamova, Summaries of Papers Presented at the Second Symposium on the Chemistry and Technology of Heterocyclic Compounds of Fossil Fuels [in Russian], Donetsk (1973), p. 4.
A. N. Kost, S. M. Gorbunova, and V. A. Budylin, Khim. Geterotsikl. Soedin., 1522 (1971).
K. Izhizumi, T. Shioiri, and S. Jamade, Chem. Pharm. Bull. (Tokyo), 15, 863 (1967); Chem. Abstr., 67, 108, 510 (1967).
A. N. Kost, S. M. Gorbunova, L. P. Basova, V. K. Kiselev, and V. I. Gorbunov, Khim.-Farmats. Zh., (1974, in press).
A. J. Namis, E. Cortes, O. Collera, and T. Walls, Boletin Institute Quimico, Univ. Nacional Mexico, 18, 64 (1966).
O. Diels and A. Köllisch, Ber., 44, 263 (1911).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 9, pp. 1229–1232, September, 1974.
Rights and permissions
About this article
Cite this article
Ginzburg, I.M., Gorbunova, S.M., Abramovich, M.A. et al. Ir spectra and conformation of 2-acetylindoles. Chem Heterocycl Compd 10, 1068–1071 (1974). https://doi.org/10.1007/BF00472124
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00472124