Abstract
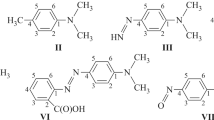
The UV, IR, and PMR spectra of N-methyl-2-aryliminolactams and their salts were examined. The ionization constants of the investigated amidines in aqueous alcohol depend substantially on the ring size (the basicities fall in the order 6 > 7 > 5). On the basis of the correlation dependences of the pK a values of the amidines and the Hammett σ constants it is shown that the π electrons of the benzene ring are conjugated both with the C=N bond and with the p electrons of the exocyclic nitrogen atom. The angles of rotation of the benzene ring with respect to the C=N bond were calculated.
Similar content being viewed by others
Literature cited
N. P. Kostyuchenko, V. G. Granik, A. M. Zhidkova, R. G. Glushkov, and Yu. N. Sheinker, Khim. Geterotsikl. Soedin., 1212 (1974).
V. G. Granik, A. M. Zhidkova, T. F. Vlasova, R. G. Glushkov, and Yu. N. Sheinker, Khim. Geterotsikl. Soedin., 533 (1974).
R. Huisgen, M. Brade, H. Walz, and G. Glogger, Ber., 90, 1437 (1957).
E. M. Peresleni, Yu. N. Sheinker, N. P. Zosimova, and Yu. I. Pomerantsev, Zh. Fiz. Khim., 39, 92 (1965).
E. M. Peresleni, V. I. Sheichenko, and Yu. N. Sheinker, Zh. Fiz. Khim., 40, 38 (1966).
Z. B. Kiro, Yu. A. Teterin, L. N. Nikolenko, and B. I. Stepanov, Zh. Organ. Khim., 8, 2573 (1972).
B. I. Stepanov, B. A. Korolev, and N. A. Rozanel'skaya, Zh. Obshch. Khim., 39, 2105 (1969).
B. M. Wepster, in: Advances in Chemistry [Russian translation], Goskhimizdat, Moscow (1961), Chap. 13.
H. B. Klevans and I. R. Platt, J. Amer. Chem. Soc., 71, 1714 (1949).
K. Maier, M. Baumann, and D. Leuchs, Chemische Zentrallblat, 132, 4525 (1961).
V. G. Granik, M. K. Polievktov, and R. G. Glushkov, Zh. Organ. Khim., 7, 1431 (1971).
A. Albert and E. Serjeant, Ionization Constants of Acids and Bases, Methuen (1964).
Author information
Authors and Affiliations
Additional information
See [1] for communication XII.
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 9, pp. 1220–1225, September, 1974.
Rights and permissions
About this article
Cite this article
Granik, V.G., Zhidkova, A.M., Glushkov, R.G. et al. Lactam acetals. Chem Heterocycl Compd 10, 1060–1064 (1974). https://doi.org/10.1007/BF00472122
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00472122