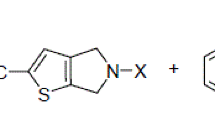
Abstract
The nucleophilic reactions of 2,3-dichlorobenzo[b]thiophene 1,1-dioxide with α- and β-glycols, α-diamines, and α-amino alcohols proceed with cleavage of hydrogen chloride to give the previously unknown 3-monosubstituted derivatives of 2-chlorobenzo[b]thiophene 1,1-dioxide. The second functional group (the hydroxyl group in amino alcohols) does not enter into the reaction.
Similar content being viewed by others
Literature cited
H. D. Hartough and S. L. Meisel, Compounds with Condensed Thiophene Rings, New York (1954), p. 156.
F. G. Bordwell, F. Ross, and J. Weinstock, J. Am. Chem. Soc., 82, 2878 (1960).
F. G. Bordwell, R. M. Hemwall, and D. A. Schexnayder, J. Org. Chem., 33, 3233 (1968).
V. I. Dronov, G. M. Prokhorov, and V. S. Fal'ko, Zh. Organ. Khim., 7, 163 (1971).
M. G. Voronkov, V. É. Udré, and É. P. Popova, Khim. Geterotsikl. Soedin., 1003 (1967).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 12, pp. 1602–1604, December, 1972.
Rights and permissions
About this article
Cite this article
Udré, V.É., Voronkov, M.G. Nucleophilic addition of difunctional reagents to 2,3-dichlorobenzo[b]thiophene 1,1-dioxide. Chem Heterocycl Compd 8, 1451–1453 (1972). https://doi.org/10.1007/BF00471826
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00471826