Abstract
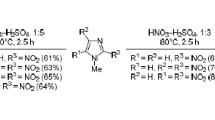
Nitration of 4-methyl-2-[2-(nitro-2-furyl)vinyl]thiazole with a mixture of concentrated nitric and sulfuric acids leads to 4-methyl-5-nitro-2-[2-(3,5-dinitro-2-furyl)vinyl]thiazole. Under the same conditions 2-methyl- and 2-acetamido-4-[1-R-2-(5-nitro-2-furyl)vinyl]thiazoles (R=CH3, Cl) are nitrated in the 3 position of the furan ring, 2-amino-4-[1-chloro-2-(5-nitro-2-furyl)vinyl]thiazole is nitrated in the 5 position of the thiazole ring and 2-acetamido-5-nitro-4-[2-(2-furyl)vinyl]thiazole undergoes profound changes. Under the influence of a mixture of of nitric acid and acetic anhydride the latter compound is converted quantitatively to the 5-nitro derivative (with respect to the furan ring), whereas 4-[2-(5-nitro-2-furyl)vinyl]thiazole derivatives do not undergo reaction.
Similar content being viewed by others
Literature cited
Dainippon Seiyoku Co., Japanese Patent No. 4983 (1963); Chem. Abstr., 65, 2278 (1966).
K. Miura, T. Oohashi, S. Matsuda, and J. Igarashi, J. Pharm. Soc. Jpn., 83, 771 (1963).
A. Fujima, J. Aritomi, S. Minami, and H. Takamatsu, J. Pharm. Soc. Jpn., 86, 427 (1966).
A. Silberg, A. Benko, and I. A. Panczel, Rev. Roumaine Chim., 10, 617 (1965).
S. B. Dickey, E. B. Towne, M. S. Bloom, W. H. Moore, H. H. Hill, H. Heynemann, D. G. Hedberg, D. C. Sievers, and U. V. Otis, J. Org. Chem., 24, 187 (1959).
P. L. Soutwick and D. I. Sapper, J. Org. Chem., 19, 1926 (1954).
K. Ingold, Theoretical Foundations of Organic Chemistry [Russian translation], Mir, Moscow (1973), p. 243.
I. Rinkes, Rec. Trav. Chim., 50, 981 (1931).
J. D. Dickey, E. B. Towne, and G. F. Wright, J. Org. Chem., 20, 499 (1955).
E. Ochiai and I. Nishizawa, J. Pharm. Japan, 59, 43 (1939); Chem. Zentralblatt, 1, 1805 (1941).
N. O. Saldabol, S. A. Giller, L. N. Alekseeva, and L. V. Kruzmetra, Khim.-farm. Zh., No. 10, 15 (1971).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 3, pp. 314–317, March, 1977.
Rights and permissions
About this article
Cite this article
Saldabol, N.O., Popelis, Y.Y. Nitration of 2- and 4-[2-(2-furyl)vinyl]thiazole derivatives. Chem Heterocycl Compd 13, 246–249 (1977). https://doi.org/10.1007/BF00470303
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00470303