Abstract
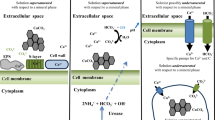
Techniques exist which allow for the measurement of in situ grazing rates of meiobenthos on sedimentary bacteria and microalgae. Radiolabeled substrates are incorporated into microbes which serve as food for meiofauna and which themselves also become labeled during feeding. However, during in situ grazing experiments, meiofauna may become radiolabeled by a variety of non-feeding processes. Proper controls to correct for these extraneous routes of labeling have been developed in the present study. The use of [methyl-3H] thymidine (3HTdR) in studies of meiofaunal grazing on bacteria has two unique advantages: (1) it is incorporated only into prokaryotic macromolecules, and (2) bacterial incorporation of 3HTdR may be selectively blocked by several inhibitors which are non-toxic to meiofaunal grazers. Coupled with formalin-killed control treatments, the use of these inhibitors makes it possible to accurately determine the partitioning of radiolabel into meiofauna during grazing into adsorptive, absorptive and grazing components. A saturated solution of nalidixic acid and 5′-deoxythymidine was found to be most effective in inhibiting water-column bacterial uptake and incorporation of 3HTdR, and had no toxic effects on meiofauna. The inhibitor was found to immediately block bacterial incorporation of 3HTdR and was as effective at 20% saturation as at 100%. The presence of sediment reduced the effectiveness of this inhibitor by 50%. Solutions of the inhibitor with excess undissolved material, however, completely blocked sediment bacterial uptake of 3HTdR. Employing these techniques during in situ grazing experiments showed that up to 83% of total meiofaunal uptake of 3H-label may be attributable to non-grazing processes. Experiments conducted in slurried sediments yielded grazing rates which were the same as those from intact cores. Furthermore, meiofaunal grazing rates on multiple food sources (e.g. bacteria and diatoms) may be determined synoptically by adding isotopically-distinct substrates (e.g. 3HTdR and H14CO3) to the same experimental incubation.
Similar content being viewed by others
Literature cited
Admiraal, W., Bouwman, L. A., Hoekstra, L., Romeyn, K. (1983). Qualitative and quantitative interactions between microphytobenthos and herbivorous meiofauna on a brackish intertidal mudflat. Int. Revue ges. Hydrobiol. 68: 175–191
Ammerman, J. W., Fuhrman, J. A., Hagström, Å., Azam, F. (1984). Bacterioplankton growth in seawater: I. Growth kinetics and cellular characteristics in seawater cultures. Mar. Ecol. Prog. Ser. 18: 31–39.
Bauer, J. E., Capone, D. G. (1985). Effects of four aromatic organic pollutants on microbial glucose metabolism and thymidine incorporation in marine sediments. Appl. envirl Microbiol. 49: 828–835
Brown, T. J., Sibert, J. R. (1977). The food of some benthic harpacticoid copepods. J. Fish. Res. Bd Can. 34: 1028–1031
Carlson, D. J., Mayer, L. M., Brann, M. L., Mague, T. H. (1985). Binding of monomeric organic compounds to macromolecular dissolved organic matter in seawater. Mar. Chem. 16: 141–153
Carman, K. R., Thistle, D. (1985). Microbial food partitioning by three species of benthic copepods. Mar. Biol. 88: 143–148
Conover, R. J., Francis, V. (1973). The use of radioactive isotopes to measure the transfer of materials in aquatic food chains. Mar. Biol. 18: 272–283
Cozzarelli, N. R. (1980). DNA gyrase and the supercoiling of DNA. Science, N.Y. 207: 953–960
Daro, M. H. (1978). A simplified 14C method for grazing measurements on natural planktonic populations. Helgoländer wiss. Meeresunters. 31: 241–248
Ducklow, H. W., Purdie, D. A., Williams Le B. P. J. (1986). Bacterioplankton: a sink for carbon in a coastal marine plankton community. Science, N.Y. 232: 865–867
Duncan, A., Schiemer, F., Klekowski, R. Z. (1974). A preliminary study of feeding rates on bacterial food by adult females of a benthic nematode, Plectus palustris DeMan, 1880. Polskie Archwm Hydrobiol. 21: 249–258
Fallon, R. D., Newell, S. Y., Hopkinson, C. S. (1983). Bacterial production in marine sediments: will cell-specific measures agree with whole-system metabolism? Mar. Ecol. Prog. Ser. 11: 119–127
Findlay, S., Meyer, J. L., Smith, P. J. (1984). Significance of bacterial biomass in the nutrition of a freshwater isopod (Lirceus sp.). Oecologia 63: 38–42
Findlay, R. H., White, D. C. (1983). Assay of polymeric betahydroxyalkanoates from environmental samples and Bacillus megaterium. Appl. envir. Microbiol. 45: 71–78
Fuhrman, J. A., Azam, F. (1980). Bacterial secondary production estimates for coastal waters of British Columbia, Antarctica, and California. Appl. envirl Microbiol. 39: 1085–1095
Fuhrman, J. A., Azam, F. (1982). Thymidine incorporation as a measure of heterotrophic bacterioplankton production in marine surface waters: evaluation and field results. Mar. Biol. 66: 109–120
Fuhrman, J. A., McManus, G. B. (1984). Do bacteria-sized marine eukaryotes consume significant bacterial production? Science, N.Y. 224: 1257–1260
Guidi, L. (1984). The effect of food consumption on ingestion, development, and survival of a harpacticoid copepod, Tisbe cucumariae Humes. J. exp. mar. Biol. Ecol. 84: 101–110
Haney, J. F. (1971). An in situ method for measurement of zooplankton grazing rates. Limnol. Oceanogr. 16: 970–977
Hollibaugh, J. T., Fuhrman, J. A., Azam, F. (1980). Radioactive labeling of natural assemblages of bacterioplankton for use in trophic studies. Limnol. Oceanogr. 25: 172–181
Jensen, L. M. (1984). Antimicrobial action of antibiotics on bacterial and algal carbon metabolism: on the use of antibiotics to estimate bacterial uptake of algal extracellular products. Arch. Hydrobiol. 99: 423–432
Jensen P. (1982). Diatom-feeding behaviour of the free-living marine nematode Chromodorita tenuis. Nematologica, 28: 71–76
Kempthorne, O., Allmaras, R. R. (1965). Errors in observation. In: C. A. Black (ed.) Methods in soil analysis. Part 1. Physical and minerological properties, including statistics of measurement and sampling. American Society of Agronomy, Madison, p. 1–23
Leach, J. H. (1970). Epibenthic algal production in an intertidal mudflat. Limnol. Oceanogr. 15: 514–521
Montagna, P. A. (1983). Live controls for radioisotope food chain experiments using meiofauna. Mar. Ecol. Prog. Ser. 12: 43–46
Montagna, P. A. (1984a). Competition for dissolved glucose between meiobenthos and sediment microbes. J. exp. mar. Biol. Ecol. 76: 177–190
Montagna, P. A. (1984b) In situ measurement of meiobenthic grazing rates on sediment bacteria and edaphic diatoms. Mar. Ecol. Prog. Ser. 18: 119–130
Moriarty, D. J. W., Pollard P. C. (1982). Diel variation of bacterial productivity in seagrass (Zostera capricorni) beds measured by the rate of thymidine incorporation into DNA. Mar. Biol. 72: 165–173
Rieper, M. (1978). Bacteria as food for marine harpacticoid copepods. Mar. Biol. 45: 337–345
Rieper, M. (1982). Feeding preferences of marine harpacticoid copepods for various species of bacteria. Mar. Ecol. Prog. Ser. 7: 303–307
Rieper, M. (1984). Relationships between bacteria and marine copepods. In: University Provence (eds.) Bacteriologie Marine. CNRS, Paris, p. 169–172
Roman, M. R., Rublee, P. A. (1981). A method to determine in situ zooplankton grazing rates on natural particle assemblages. Mar. Biol. 65: 303–309
Romeyn, K., Bouwman, L. A. (1983). Food selection and consumption by estuarine nematodes. Hydrobiol. Bull. 17: 103–109
Sellner, B. W. (1976). Survival and metabolism of the harpacticoid copepod Thompsonula hyaenae (Thompson) fed on different diatoms. Hydrobiologia 50: 233–238
Van den Berghe, W., Bergmans, M. (1981). Differential food preferences in three co-occurring species of Tisbe (Copepoda, Harpacticoida). Mar. Ecol. Prog. Ser. 4: 213–219
Wright, H. T., Nurse, Y. C., Goldstein, D. J. (1981). Nalidixic acid, oxolinic acid and novobiocin inhibit yeast glycyl- and leucyltransfer RNA synthetases. Science, N.Y. 213: 455–456
Yetka, J. E., Wiebe, W. J. (1974). Ecological application of antibiotics as respiratory inhibitors of bacterial populations. Appl. Microbiol. 28: 1033–1039
Zobell, C. E., Feltham, C. B. (1938). Bacteria as food for certain marine invertebrates. J. mar. Res. 1: 312–327
Author information
Authors and Affiliations
Additional information
Communicated by R.S. Carney, Baton Rouge
University of Texas Marine Science Contribution No. 698.
Rights and permissions
About this article
Cite this article
Montagna, P.A., Bauer, J.E. Partitioning radiolabeled thymidine uptake by bacteria and meiofauna using metabolic blocks and poisons in benthic feeding studies. Marine Biology 98, 101–110 (1988). https://doi.org/10.1007/BF00392664
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00392664