Abstract
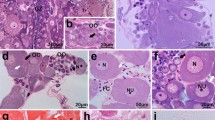
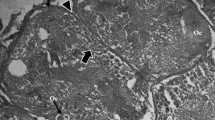
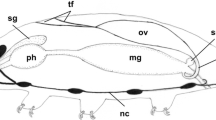
Ultrastructural features of oogenesis were examined in the pelagic polychaetes Rhynchonerella angelini and Alciopa reynaudii which were collected from Bahamian waters by a manned submersible during 1979 and 1980. No definitive ovary was detected in either species. Oogonia are released into the coelom as packets of cells, where they undergo mitotic division while surrounded by an envelope of sheath cells. Cytokinesis is incomplete, resulting in intercellular bridges between oogonia. Oocytes undergo early stages of meiosis characterized by the presence of synapsed chromosomes, followed by a period of rapid cytoplasmic and nuclear growth. Oocytes are released from the packets in the early vitellogenic phase into the coelom, where they undergo yolk synthesis as solitary coelomic cells. Vitellogenesis includes both autosynthetic and heterosynthetic processes. Autosynthesis involves the fusion of secretory vesicles formed by the combined activity of the rough endoplasmic reticulum and Golgi complex, with convoluted electron-dense tubular bodies of unknown origin. Heterosynthesis involves the intense uptake of exogenous precursors through endocytosis and their fusion into nascent yolk bodies which, in turn, are presumed to fuse with autosynthetically-derived yolk bodies. No nutrient stores were detected in somatic tissues. Early and middle stages of vitellogenic oocytes were absent from the coelom. This absence combined with the high level of endocytotic activity suggests that vitellogenesis occurs rapidly. These features, in combination with the presence of an exceptionally thin body wall and gut, might serve as related adaptations for predator avoidance by the maintenance of relatively low tissue-density. Alciopid, phyllodocid, and nereid polychaetes share some common reproductive features including the presence of “dispersed” ovaries, clusters of syncytial germ cells which undergo meiosis while enveloped by somatic cells and the release of oocytes from the clusters prior to vitellogenesis.
Similar content being viewed by others
Literature cited
Anderson, E. (1974). Comparative aspects of the ultrastructure of the female gamete. Int. Rev. Cytol. (Suppl) 4: 1–70
Anderson, E., Huebner E. (1968). Development of the oocyte and its accessory cells of the polychaete Diopatra cuprea (Bosc.). J. Morph. 126: 163–172
Audouin, J. V., Milne-Edwards, H. (1833). Classification des annélides et description de celles qui habitent les côtes de la France. Annls Sci. nat. 29: 195–269
Cason, J. E. (1950). A rapid one-step Mallory-Heidenhain stain for connective tissue. Stain Technol. 25: 225–226
Christie, G. (1984). The reproductive biology of a Northumberland population of Sphaerodorum gracilis (Rathke, 1843) (Polychaeta: Sphaerodoridae). Sarsia 69: 117–121
Christie, G. (1985). A comparative study of the reproductive cycles of three Northumberland populations of Chaetozone setosa (Polychaeta: Cirratulidae). J. mar. biol. Ass. U.K. 65: 239–254
Dales, R. P. (1955). The evolution of the pelagic alciopid and phylodocid polychaetes. Proc. zool. Soc. Lond. 125: 411–420
Dales, R. P. (1955). Pelagic polychaetes of the Pacific Ocean. Bull. Scripps Instn Oceanogr. 7: 95–167
Dales, R. P., Peter, G. (1972). A synopsis of the pelagic Polychaeta. J. nat. Hist. 6: 55–92
Dhainaut, A. (1967). Étude de la vitellogenèse chez Nereis diversicolor O. F. Müller (annélide polychète) par autoradiographie à haute résolution. C.r. hebd. Séanc. Acad. Sci. Paris 265D: 434–436
Dhainaut, A. (1970). Étude cytochimique et ultrastructurale de l'évolution ovocytaire de Nereis pelagica L. (annélide polychète) I. Ovogenèse naturelle. Z. Zellforsch. mikrosk. Anat. 104: 375–390
Dhainaut, A. (1972). Évolution nucléolaire au cours de l'ovogenèse de Nereis pelagica. I. Étude morphologique. J. Microscopie (Paris) 13: 67–84
Dhainaut, A., Porchet, M., Fischer, A., Baert, J. L. (1984). Biochemical and metabolic aspects of oocyte differentiation in nereids (Annelida, Polychaeta). Adv. Invertebrate Reprod. 3: 3–16
Eckelbarger, K. J. (1975). A light and electron microscope investigation of gametogenesis in Nicolea zostericola (Polychaeta: Terebellidae). Mar. Biol. 30: 353–370
Eckelbarger, K. J. (1980). An ultrastructural study of oogenesis in Streblospio benedicti (Spionidae), with remarks on diversity of vitellogenic mechanisms in Polychaeta. Zoomorphologie 94: 241–263
Eckelbarger, K. J. (1983). Evolutionary radiation in polychaetes ovaries and vitellogenic mechanisms and their role in life history patterns. Can. J. Zool. 61: 487–504
Eckelbarger, K. J. (1984) Comparative aspects of oogenesis in polychaetes. Fortschr. Zool. 29: 123–148
Eckelbarger, K. J. (1986) Vitellogenic mechanisms and the allocation of energy to offspring in polychaetes. Bull. mar. Sci. 39: 426–443
Eckelbarger, K. J. (In press). Oogenesis and female gametes. In: Hermans, C. O., Westheide, W. (eds.) The ultrastructure of the Polychaeta. Springer-Verlag, Heidelberg
Emanuelsson, H. (1969). Electronmicroscopical observations on yolk and yolk formation in Ophryotrocha labronica La Greca and Bacci. Z. Zellforsch. mikrosk. Anat. 95: 19–36
Fauchald, K. (1977). The polychaete worms. Definitions and keys to the orders, families and genera. Sci. Ser. nat. Hist. Mus. Los Ang. Cty 28: 1–190
Fawcett, D. W. (1961). Intercellular bridges. Expl Cell Res. (Suppl.) 8: 174–187
Fischer, A. (1974). Stages and stage distribution in early oogenesis in the annelid, Platynereis dumerilii. Cell Tissue Res. 156: 35–45
Fischer, A. (1975). The structure of symplasmic early oocytes and their enveloping sheath cells in the polychaete, Platynereis dumerilii. Cell Tissue Res. 160: 327–343
Fischer, A., Dhainaut, A. (1985). The origin of yolk in the oocytes of Nereis virens (Annelida, Polychaeta). Cell Tissue Res. 240: 67–76
Heacox, A. E., Schroeder, P. C. (1981). A light and electron microscopic investigation of gametogenesis in Typosyllis pulchra (Berkeley and Berkeley) (Polychaeta: Syllidae). II. Oogenesis. Cell Tissue Res. 218: 641–658
Hermans, C. O., Eakin, R. M. (1974). Fine structure of the eyes of and alciopid polychaete, Vanadis tangensis (Annelida). Z. Morph. Tiere 79: 245–267
Hutchings, P. A. (1973). Age structure and spawning of a Northumberland population of Melinna cristata (Polychaeta: Ampharetidae). Mar. Biol. 18: 218–227
Kinberg, J. G. H. (1866). Annulata nova. Öfvers. K. VetenskAkad. Förh 22: 167–179
Klesch, W. L. (1970). The reproductive biology and larval development of Laeonereis culveri (Webster) (Polychaeta, Nereidae). Contr. mar. Sci. Univ. Tex. 15: 71–85
Muus, B. J. (1967). The fauna of Danish estuaries and lagoons. Meddr. Danm. Fisk.-og Havunders. 5: 1–316
Olive, P. J. W. (1971). Ovary structure and oogenesis in Cirratulus cirratus (Polychaeta: Cirratulidae). Mar. Biol. 8: 243–259
Olive, P. J. W. (1975a). Reproductive biology of Eulalia viridis (Müller) (Polychaeta: Phyllodocidae) in the northeastern U.K. J. mar. biol. Ass. U.K. 55: 313–336
Olive, P. J. W. (1975b) Germinal epithelium activity in polychaetes in relation to periodicity of reproduction. Pubbl. Staz. zool. Napoli (Suppl.) 39: 267–281
Olive, P. J. W. (1977). Observations on the productive cycles, gametogenesis and endocrinology of the polychaetes, Eulalia viridis (Phyllodocidae) and Nephtys hombergi (Nephtyidae). In: Adiyodi, K. G., Adiyodi, R. G. (eds.) Advances in invertebrate reproduction, Vol. 1. Karivellur, Kerala, India, International Society for Invertebrate Reproduction, Peralam-Kenoth, p. 389–403
Olive, P. J. W. (1983). Oogenesis in Annelida: Polychaeta. In: Adiyodi, K. G., Adiyodi, R. G. (eds.) Reproductive biology of invertebrates: a multivolume treatise, Vol. 1, Oogenesis, oviposition, and oosorption. Wiley, New York, p. 357–422
Paxton, H. (1979). Taxonomy and aspects of the life history of Australian beachworms (Polychaeta: Onuphidae). Aust. J. mar. Freswat. Res. 30: 265–294
Pfannenstiel, H.-D. (1978). Die Entwicklung der Kontaktstruktur von Ei und Nährzelle im Zuge der Oogenese von Ophryotrocha puerilis Claparède and Mecznikow (Polychaeta, Dorvilleidae). Zoomorphologie 90: 181–196
Porchet, M. (1984). Biochemistry of oocyte differentiation in nereids. Fortschr. Zool. 29: 207–226
Rice, S. A. (1980). Morphology, reproduction and behaviour of alciopid polychaetes. Am. Zool. 20: p. 752
Rice, S. A. (1984). Reproductive biology and systematics of the Alciopidae (Polychaeta). Am. Zool. 24: p. 42A
Rice, S. A. (1987). Reproductive biology, systematics and evolution in the polychaete family Alciopidae. Bull. biol. Soc. Wash. 7: 85–96
Schroeder, P. C. (1966). A histological and autoradiographic study of normal and induced metamorphosis in the nereid polychaete Nereis grubei (Kinberg). Ph.D. dissertation. Stanford University, California
Stöp-Bowitz, C. (1948). Polychaeta from the Michael Sars North-Atlantic Deep-Sea Expedition, 1910. Rep. scient. Results Michael Sars N. Atlant. deep Sea Exped. 5: 1–91
Tweedell, K. S. (1966) Oocyte development and incorporation of 3H-thymidine and 3H-uridine in Pectinaria (Cistenides) gouldii. Biol. Bull. mar. biol. Lab., Woods Hole 131: 516–538
Ushakov, P. V. (1972). Polychaetes, Vol. 1 Fauna SSSR 102: 1–259
Wald, G., Rayport, S. (1977) Vision in annelid worms. Science, N.Y. 196: 1434–1439
Wild, A. E. (1980). Coated vesicles: a morphologically distinct subclass of endocytotic vesicles. In: Ockelford, C. D., Whyte, A. (eds.) Coated vesicles. Cambridge University Press, Cambridge, p. 1–24
Author information
Authors and Affiliations
Additional information
Communicated by J. M. Lawrence, Tampa
Rights and permissions
About this article
Cite this article
Eckelbarger, K.J., Rice, S.A. Ultrastructure of oogenesis in the holopelagic polychaetes Rhynchonerella angelini and Alciopa reynaudii (Polychaeta: Alciopidae). Marine Biology 98, 427–439 (1988). https://doi.org/10.1007/BF00391119
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00391119