Abstract
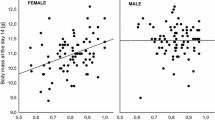
Heterozygosity and growth rate have been correlated in many molluscs, although the phenomenon is not universal. Enhanced growth of heterozygous molluscs has been attributed to lower energetic requirements for basal metabolism. We have investigated heterozygote deficiency, and the correlation between single-locus and multiple-locus heterozygosity and phenotype in juveniles of the scallop Placopecten magellanicus (Gmelin). Six samples were collected between 22 November 1984 and 11 December 1985 at Passamaquoddy Bay, New Brunswick, Canada. On average, heterozygote deficiency was small, although somewhat higher at the octopine dehydrogenase (Odh) locus, and decreased with age. No correlation was observed between genotype and growth rate. This result and published records indicate that allozyme heterozygosity of pectinids does not influence growth to the same degree as in other bivalves. However, we have observed in P. magellanicus a correlation between allozyme heterozygosity and octopine accumulation, a trait that relates to functional anaerobiosis, and may be related to the scallop's scope for movement. We combine these results and results from the literature into a model that relates the hypothesis of “associative overdominance” (at the population genetics level) with the hypothesis of “energetic efficiency” (at the physiological level). The model assumes that energy savings attributed to heterozygosity are used to maximize fitness. In freely moving molluscs this results in enhanced activity (such as searching for prey or swimming away from a predator), and in sessile molluscs either in enhanced somatic growth in juveniles or gonadal growth in adults.
Similar content being viewed by others
Literature cited
Ayala, F. J., Powell, J. R., Tracey, M. L., Mourão, C. A., Pérez-Salas, S. (1972). Enzyme variability in the Drosophila willistoni group. IV. Genetic variation in natural populations of Drosophila willistoni. Genetics 70: 113–139
Beaumont, A. R. (1982). Variations in heterozygosity at two loci between year classes of a population of Chlamys opercularis (L.) from a Scottish sea-loch. Marine Biology Lett. 3: 25–33
Beaumont, A. R., Beveridge, C. M. (1984). Electrophoretic survey of genetic variation in Pecten maximus Chlamys opercularis, C. varia and C. distorta from the Irish Sea. Marine Biology 81: 299–306
Beaumont, A. R., Budd, M. D. (1983). Effects of self-fertilisation and other factors on the early development of the scallop Pecten maximus. Marine Biology 76: 285–289
Beaumont, A. R., Gosling, E. M., Beveridge, C. M., Budd, M. D., Burnell, G. M. (1985). Studies on heterozygosity and size in the scallop, Pecten maximus. In: Gibbs, P. E. (ed.) Proc. 19th Eur. Marine Biology Symp., Cambridge University Press, Cambridge, p. 443–454
Berger, E. (1976). Heterosis and the maintenance of enzyme polymorphism. Am. Nat. 110: 823–839
Bulnheim, H.-P., Gosling, E. (1988). Population genetic structure of mussels from the Baltic Sea. Helgoländer Meeresunters. 42: 113–129
Dadswell, M. J., Chantler, R. A., Parsons, G. J. (1987). Spat settlement and early growth of giant scallop, Placopecten magellanicus in Passamaquoddy Bay and the Bay of Fundy, Canada. The sixth international pectinid Workshop, Menai Bridge, Wales, 9 – 14 April 1987. Int. Counc. Explor. Sea. Comm. Meet. (Shellfish and Benthos Comm.) K:3:1–23
Diehl, W. J., Gaffney, P. M., Koehn, R. K. (1986). Physiological and genetic aspects of growth in the mussel Mytilus edulis. I. Oxygen consumption, growth and weight loss. Physiol. Zool. 59:201–211
Diehl, W. J., Koehn, R. K. (1985). Multiple-locus heterozygosity, mortality, and growth in a cohort of Mytilus edulis. Marine Biology 88:265–271
Foltz, D. W. (1986). Null alleles as possible cause of heterozygote deficiencies in the oyster Crassostrea virginica and other bivalves. Evolution 40: 869–870
Foltz, D. W., Zouros, E. (1984). Enzyme heterozygosity in the scallop Placopecten magellanicus (Gmelin) in relation to age and size. Marine Biology Lett. 5: 255–263
Gaffney, P. M., Scott, T. M. (1984). Genetic heterozygosity and production traits in natural and hatchery populations of bivalves. Aquaculture, Amsterdam 42: 289–302
Gartner-Kepkay, K. E., Dickie, L. M., Freeman, K. R., Zouros, E. (1980). Genetic differences and environments of mussel populations in the maritime provinces. Can. J. Fish. aquat. Sci. 37: 775–782
Gartner-Kepkay, K. E., Zouros, E. (1985). Influence of environment and human selection on the genetic structure of some economically important marine animal species. Final report: Department of Supply and Services Contract (D.F.O., Halifax, N.S., Canada), File No 08sc.FP101-3-0301
Gartner-Kepkay, K. E., Zouros, E., Dickie, L. M., Freeman, K. R. (1983). Genetic differentiation in the face of gene flow: a study of mussel populations from a single Nova Scotia embayment. Can. J. Fish. aquat. Sci. 40: 443–451
Carton, D. W. (1984). Relationship between multiple locus heterozygosity and physiological energetics of growth in the estuarine gastropod Thais haemastoma. Physiol. Zool. 57: 530–543
Garton, D. W., Koehn, R. K., Scott, T. M. (1984). Multiple-locus heterozygosity and the physiological energetics of growth in the coot clam, Mulinia lateralis, from a natural population. Genetics 108: 445–455
Gentili, M. R., Beaumont, A. R. (1988). Environmental stress, heterozygosity, and growth rate in Mytilus edulis L. J. exp. Marine Biology Ecol. 120: 145–153
Gosling, E. M. (1989). Genetic heterozygosity and growth rate in a cohort of Mytilus edulis from the Irish coast. Marine Biology 100: 211–215
Hawkins, A. J. S., Bayne, B. L., Day, A. J. (1986). Protein turnover, physiological energetics and heterozygosity in the blue mussel, Mytilus edulis: the basis of variable age-specific growth. Proc. R. Soc. (Ser. B) 229: 161–176
Hawkins, A. J. S., Bayne, B. L., Day, A. J. Rusin, J., Worrall, C. M. (in press). Genotype-dependent protein metabolism and fitness in Mytilus edulis. In: Proc. 23rd Eur. Marine Biology Symp. 1988
Holley, M. E., Foltz, D. W. (1987). Effects of multiple-locus heterozygosity and salinity on clearance rate in a brackish-water clam, Rangia cuneata (Sowerby). J. exp. Marine Biology Ecol. 111: 121–131
Hvilsom, M. M., Theisen, B. F. (1984). Inheritance of allozyme variations through crossing experiments in the blue mussel, Mytilus edulis L. Hereditas 101: 1–7
Koehn, R. K., Diehl, W. J., Scott, T. M. (1988). The differential contribution of individual enzymes of glycolysis and protein catabolism to the relationship between heterozygosity and growth rate in the coot clam, Mulinia lateralis. Genetics 118: 121–130
Koehn, R. K., Gaffney, P. M. (1984). Genetic heterozygosity and growth rate in Mytilus edulis. Marine Biology 82: 1–7
Koehn, R. K., Milkman, R., Mitton, J. (1976). Population genetics of marine pelecypods. IV. Selection, migration and genetic differentiation in the blue mussel Mytilus edulis. Evolution 30: 2–32
Koehn, R. K., Shumway, S. E. (1982). A genetic/physiological explanation for differential growth rate among individuals of the American oyster, Crassostrea virginica (Gmelin). Marine Biology Lett. 3: 35–42
Langton, R. W., Robinson, W. E., Schick, D. (1987). Fecundity and reproductive effort of sea scallops Placopecten magellanicus from the Gulf of Maine. Mar. Ecol. Prog. Ser. 37: 19–25
MacDonald, B. A., Thompson, R. J., Bayne, B. L. (1987). Influence of temperature and food availability on the ecological energetics of the giant scallop Placopecten magellanicus. IV. Reproductive effort, value and cost. Oecologia (Berl.) 72: 550–556
Mallet, A. L., Zouros, E., Gartner-Kepkay, K. E., Freeman, K. R., Dickie, L. M. (1985). Larval viability and heterozygote deficiency in populations of marine bivalves: evidence from pair matings of mussels. Mar. Biol 87: 165–172
Mitton, J. B., Grant, M. C. (1984). Associations among protein heterozygosity, growth rate and developmental homeostasis. A. Rev. Ecol. Syst. 15: 479–499
Ohta, T. (1971). Associative overdominance caused by linked detrimental mutations. Genet. Res. 18: 277–286
Rodhouse, P. G., Gaffney, P. M. (1984). Effect of heterozygosity on metabolism during starvation in the American oyster Crassostrea virginica. Marine Biology 80: 179–187
Rodhouse, P. G., McDonald, J. H., Newell, R. I. E., Koehn, R. K. (1986). Gamete production, somatic growth and multiple-locus enzyme heterozygosity in Mytilus edulis. Marine Biology 90: 209–214
Schaal, B. A., Anderson, W. W. (1974). An outline of techniques for starch gel electrophoresis of enzymes from the American oyster Crassostrea virginica Gmelin. Ga. Mar. Sci. Cen., Tech. Rep. Ser. 74. 1–18
Selander, R. K., Smith, M. H., Yang, S. Y., Johnson, W. E., Gentry, J. B. (1971). Biochemical polymorphism and systematics in the genus Peroyscus. I. Variation in the old-field mouse (Peromyscus polionotus). Stud. Genet., Austin, Tex. 6: 49–90
Siebenaller, J. F. (1979). Octopine dehydrogenase (E.C. 1.5.1.a) polymorphism in Mytilus edulis. Isozyme Bull. 12: 67–68
Singh, S. M. (1982). Enzyme heterozygosity associated with growth at different developmental stages in oysters. Can. J. Genet. Cytol. 24: 451–458
Singh, S.M., Green, R. H. (1984). Excess of allozyme homozygosity in marine molluscs and its possible biological significance. Malacologia 25: 569–581
Singh, S. M., Zouros, E. (1978). Genetic variation associated with growth rate in the American oyster (Crassostrea virginica). Evolution 32: 342–353
Sokal, R. R., Rohlf, F. J. (1981). Biometry. W. H. Freeman, New York
Volckaert, F. (1988). The implications of heterozygosity in the scallop Placopecten magellanicus (Gmelin). Ph. D. thesis, Dalhousie University, Halifax, Canada
Waterlow, J. C., Jackson, A. A. (1981). Nutrition and protein turnover in man. Br. Med. Bull. 37: 5–10
Wilkins, N. P. (1978). Length-correlated changes in heterozygosity at an enzyme locus in the scallop (Pecten maximus L.). Anim. Blood Grps biochem. Genet. 9: 69–77
Zouros, E. (1978). On the relation between heterozygosity and heterosis: an evaluation of the evidence from marine molluscs. Isozymes: current topics in biological and medical research. 15: 255–270
Zouros, E., Foltz, D. W. (1984a). Possible explanations of heterozygote deficiency in bivalve molluscs. Malacologia 25: 583–591
Zouros, E., Foltz, D. W. (1984b). Minimal selection requirements for the correlation between heterozygosity and growth, and for the deficiency of heterozygotes, in oyster populations. Dev. Genet. 4: 393–405
Zouros, E., Foltz, D. W. (1987). The use of allelic isozyme variation for the study of heterosis. Isozymes: current topics in biological and medical research. 13: 1–59
Zouros, E., Mallet, A. L. (in press). Genetic explantations of the growth/heterozygosity correlation in marine molluscs. Proc. 23rd Eur. Marine Biology Symp. 1988
Zouros, E., Romero-Dorey, M., Mallet, A. L. (1988). Heterozygosity and growth in marine bivalves: further data and the associative overdominance explantation. Evolution 42: 1332–1341
Zouros, E., Singh, S. M., Miles, H. E. (1980). Growth rate in oysters: an overdominant phenotype and its possible explanations. Evolution 34: 856–867
Author information
Authors and Affiliations
Additional information
Communicated by O. Kinne, Oldendorf/Luhe.
Rights and permissions
About this article
Cite this article
Volckaert, F., Zouros, E. Allozyme and physiological variation in the scallop Placopecten magellanicus and a general model for the effects of heterozygosity on fitness in marine molluscs. Marine Biology 103, 51–61 (1989). https://doi.org/10.1007/BF00391064
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00391064