Abstract
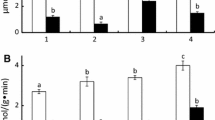
A procedure for the determination of chitinase activity was adapted for the seawater environment. Preliminary data indicate that the controlled bacterial environment within the digestive tracts of marine fishes and possibly other marine animals plays a significant role in the decomposition and recycling of chitin. It is estimated in the stomachs of a single population of Enophrys bison (buffalo sculpin) of 1×105 fish that ca. 16 metric tons of chitin could be decomposed annually.
Similar content being viewed by others
Literatuce Cited
Barrington, E.J.W.: The alimentary canal and digestion. In: The physiology of fishes, Vol. 1. pp 109–161. Ed. by M.F. Brown. New York: Academic Press 1957
Beardsley, A.J.: Movement and angler use of four foodfishes in Yaquina Bay, 173 pp. Ph.D. dissertation, Corvallis: Oregon State University 1969
Chan, J.G.: The occurrence, taxonomy and activity of chitinoclastic bacteria from sediment, water and fauna of Puget Sound, 312 pp. Ph.D. dissertation, Seattle: University of Washington 1970
Goodrich, T.D. and R.Y. Morita: Bacterial chitinase in the stomachs of marine fishes from Yaquina Bay, Oregon, USA. Mar. Biol. 41, 355–360 (1977)
Hood, M.A. and S.P. Meyers: The biology of aquatic chitinoclastic bacteria and their chitinolytic activities. La Mer (Bull. Soc. francojap. Océanogr.) 11, 25–41 (1973)
Jerde, C.W. and R. Lasker: Molting of euphausiid shrimps: shipboard observations. Limnol. Oceanogr. 11, 120–124 (1966)
Jeuniaux, C.: Chitinases. Meth. Enzym. 8, 644–650 (1966)
—: Chitinous structures. In: Comprehensive biochemistry, Vol. 26C. pp 595–632. Amsterdam: Elsevier 1971
Johnstone, J.: Conditions of life in the sea, 332 pp. Cambridge: University Press 1908
Liston, J., W.J. Wiebe and B. Lighthart: Activities of marine benthic bacteria. Contr. Univ. Wash. College (Sch.) Fish. 184, 39–41 (1965)
Morgan, W. and L. Elson: A colorimetric method for the determination of N-acetyl-glucosamine and N-acetyl-chondrosamine. Biochem. J. 28, 988–995 (1934)
Reissig, J.L., J.L. Strominger and L.F. Leloir: A modified colorimetric method for the estimation of N-acetyl-amino sugars. J. biol. Chem. 217, 959–966 (1955)
Seki, H.: Microbiological studies on the decomposition of chitin in marine environments IX. Rough estimation on chitin decomposition in the ocean. J. oceanogr. Soc. Japan 21, 261–269 (1965)
Skerman, V.B.D.: Methods. In: A guide to the identification of the genera of bacteria, p. 254. Ed. by V.B.D. Skerman. Baltimore: Williams & Wilkins Co. 1967
Author information
Authors and Affiliations
Additional information
Communicated by J.S. Pearse, Santa Cruz
Published as Technical Paper No. 4434, Oregon Agricultural Experiment Station. This research was supported by NIH training grant 5TO1 GM-00704 and NSF grant DES73-06611 AO2.
Rights and permissions
About this article
Cite this article
Goodrich, T.D., Morita, R.Y. Incidence and estimation of chitinase activity associated with marine fish and other estuarine samples. Mar. Biol. 41, 349–353 (1977). https://doi.org/10.1007/BF00389100
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00389100