Abstract
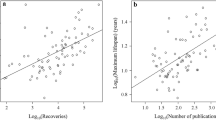
It has been proposed that antioxidants can be longevity determinants in animals. However, no comprehensive study has been conducted to try to relate free radicals with maximum life span. This study compares the lung tissue of various vertebrate species — amphibia, mammals and birds — showing very different and well known maximum life spans and life energy potentials. The lung antioxidant enzymes superoxide dismutase, catalase, Se-dependent and non-Se-dependent glutathione peroxidases, and glutathione reductase showed significantly negative correlations with maximum life span. The same was observed for the lung antioxidants, reduced glutathione and ascorbate. It is concluded that a generalized decrease in tissue antioxidant capacity is a characteristic of longevous species. It is suggested that a low rate of free radical recycling (free-radical generation and scavenging) can be an important factor involved in the evolution of high maximum animal longevities. A low free-radical production could be responsible for a low rate of damage at critical sites such as mitochondrial DNA.
Similar content being viewed by others
Abbreviations
- CAT:
-
catalase
- COX:
-
cytochrome oxidase
- GPx:
-
glutathione peroxidase
- GR:
-
glutathione reductase
- GSH:
-
reduced glutathione
- GSSG:
-
oxidized glutathione
- LEP:
-
life energy potential
- MDA:
-
malondialdehyde
- MLSP:
-
maximum life span
- MR:
-
metabolic rate
- MW:
-
molecular weight
- PO2 :
-
partial pressure of oxygen
- SOD:
-
superoxide dismutase
- VO2 :
-
basal oxygen consumption
References
Allen RG, Farmer KJ, Sohal RS (1983) Effect of catalase inactivation on levels of inorganic peroxides, superoxide dismutase, glutathione, oxygen consumption and life span in adult house-flies (Musca domestica). Biochem J 216:503–506
Altman PL, Dittmer DS (1972) Life spans: animals. In: Altman PL, Dittmer DS (eds) Biology data book, 2nd edn, vol 1. Fed Am Soc Exp Biol, Bethesda, pp 229–235
Altman PL, Dittmer DS (1974) Biology data book. In: Altman PL, Dittmer DS (eds) Biology data book, 2nd edn, vol 3. Fed Am Soc Exp Biol, Bethesda, pp 1804–1814
Ames BN (1989) Endogenous oxidative DNA damage, aging, and cancer. Free Rad Res Comms 7:121–128
Barja de Quiroga G, López-Torres M, Pérez-Campo R, Rojas C (1991) Simultaneous determination of two antioxidants, uric and ascorbic acid in animal tissue by high-performance liquid chromatography. Anal Biochem 199:81–85
Beers RF, Sizer WI (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140
Bull AW, Marnett LJ (1985) Determination of malondialdehyde by ion-pairing high-performance liquid chromatography. Anal Biochem 149:284–290
Cutler RG (1984a) Antioxidants, aging and longevity. In: Pryor WA (ed) Free radicals in biology, vol VI. Academic Press, New York, pp 371–428
Cutler RG (1984b) Urate and ascorbate: their possible roles as antioxidants in determining longevity of mammalian species. Arch Gerontol Geriatr 3:321–348
Cutler RG (1986) Aging and oxygen radicals. In: Taylor AE et al. (eds) Physiology of oxygen radicals. Amer Physiol Soc, Bethesda, pp 251–285
Cutler RG (1991) Antioxidants and aging. Am J Clin Nutr 53:373S-379S
De Marchena O, Guarnieri M, McKhann G (1974) Glutathione peroxidase levels in brain. J Neurochem 22:773–776
Duellman WE, Trueb L (1986) Population biology. In: Biology of amphibians. McGraw-Hill, New York, pp 264–265
Flower SS (1925) Contributions to our knowledge of the duration of life in vertebrate animals. IV. Birds, vol. 1925. Proc Zool Soc London, pp 1378–1404
Gey KF (1990) Lipids, lipoproteins and antioxidants in cardiovascular dysfunction. Biochem Soc Trans 18:1041–1045
Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106:207–212
Halliwell B, Gutteridge JMC (1989) Free radicals as useful species. In: Halliwell B, Gutteridge JMC (eds) Free radicals in biology and medicine. Oxford University Press, Oxford, pp 366–415
Harman D (1956) Aging: a theory based on free radical and radiation chemistry. J Gerontol 11:298–300
Kirk JE (1962) Variations in tissue content of vitamins and hormones. Vitam Horm 20:82–92
Lawrence RA, Burk RF (1976) Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 71:952–958
Lawrence RA, Burk RF (1978) Species, tissue and subcellular distribution of non Se-dependent glutathione peroxidase activity. J Nutr 108:211–215
Lidstedt SL, Calder WA (1976) Body size and longevity in birds. Condor 78:91–94
López-Torres M, Pérez-Campo R, Barja de Quiroga G (1991) Effect of natural ageing and antioxidant inhibition on liver antioxidant enzymes, glutathione system, peroxidation, and oxygen consumption in Rana perezi. J Comp Physiol B 160:655–661
López-Torres M, Pérez-Campo R, Rojas C, Cadenas S, Barja de Quiroga G (1993) Simultaneous induction of SOD, glutathione reductase, GSH and ascorbate in liver and kidney correlates with survival during aging. Free Rad Biol Med 15:133–142
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Massey V, Williams CH (1965) On the reaction mechanism of yeast glutathione reductase. J Biol Chem 240:4470–4481
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 276:368–374
Paoletti F, Aldinucci D, Mocali A, Caparrini A (1986) A sensitive spectrophotometric method for the determination of superoxide dismutase activity in tissue extracts. Anal Biochem 154:536–541
Patón D, Juarranz A, Sequeros E, Pérez-Campo R, López-Torres M, Barja de Quiroga G (1991) Seasonal age and sex structure of Rana perezi assessed by skeletochronology. J Herpetol 25:389–394
Pearl R (1928) The rate of living. University of London Press, London
Pérez R, López M, Barja de Quiroga G (1991) Aging and lung antioxidant enzymes, glutathione, and lipid peroxidation in the rat. Free Rad Biol Med 10:35–39
Pérez-Campo R, López-Torres M, Rojas C, Cadenas S, Baraa de Quiroga G catalase-depleted frogs correlates with early survival throughout the life span. Mech Ageing Dev 67:115–127
Rogers JB (1950) The development of senility in the guinea pig. Anat Rec 106:286–287
Rubner M (1908) Das Problem der Lebensdauer und seine Beziehungen zu Wachstum und Ernährung. Oldenburg R, München
Smith L (1955) Spectrophotometric assay of cytochrome c oxidase. In: Glick D (ed) Methods of biochemical analysis, vol 2. Wiley New York, pp 427–434
Sohal RS, Farmer KJ, Allen RG, Ragland SS (1984) Effects of diethyldithiocarbamate on lifespan, metabolic rate, superoxide dismutase, catalase, inorganic peroxides and glutathione in the adult male housefly, Musca domestica. Mech Ageing Dev 24:75–183
Sohal RS, Svensson I, Sohal BH, Brunk UT (1989) Superoxide anion radical production in different species. Mech Ageing Dev 53:129–135
Sohal RS, Sohal BH, Brunk UT (1990a) Relationship between antioxidant defenses and longevity in different mammalian species. Mech Ageing Dev 53:217–227
Sohal RS, Svensson I, Brunk UT (1990b) Hydrogen peroxide production by liver mitochondria in different species. Mech Ageing Dev 53:209–215
Tietze F (1969) Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 27:502–522
Tolmasoff JM, Ono T, Cutler RG (1980) Superoxide dismutase: correlation with life-span and specific metabolic rate in primate species. Proc Natl Acad Sci USA 77:2777–2781
Turrens JF, Freeman BA, Crapo JD (1983) Enhancement of O2 and H2O2 production by lung mitochondria and microsomes during hyperoxia. In: Greenwald RA, Cohen G (eds) Oxy radicals and their scavenger systems, vol II. Cellular and medical aspects. Elsevier, Amsterdam, pp 365–370
Williams RJP (1985) The necessary and the desirable production of radicals in biology. Phil Trans R Soc Lond 311:593–603
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pérez-Campo, R., López-Torres, M., Rojas, C. et al. Longevity and antioxidant enzymes, non-enzymatic antioxidants and oxidative stress in the vertebrate lung: a comparative study. J Comp Physiol B 163, 682–689 (1994). https://doi.org/10.1007/BF00369520
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00369520