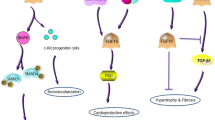
Abstract
Since the discovery of transforming growth factor-ß:s an increasing number of different biological effects have been attributed to this group of proteins. Analysis of the cellular responses to TGFß stimulation at the molecular level has indicated that TGFß acts as an activator of transcription of several genes. This may in part explain the plethora of various functions that have been ascribed to TGFß. In addition to the TGFß family of polypeptides there is an increasing number of related factors, whose major roles appear to be involved in developmental processes. A distinct feature of TGFß is its ability to regulate pericellular proteolysis of cultured cells. As yet this property has not been associated with other members of this group of polypeptides. Depending on the target cell type TGFß may either increase or decrease pericellular proteolytic activity. Proteolytic activation of latent TGFß and its possible inhibition by TGFß-induced protease inhibitors could be a physiological feed-back mechanism in the control of proteolytic activity in the vicinity of cells.
Similar content being viewed by others
Abbreviations
- GM-CSF:
-
Granulocyte-Monocyte Colony Stimulating Factor
- EGF:
-
Epidermal Growth Factor
- IL:
-
Interleukin
- MIS:
-
Müllerian Inhibitory Substance
- NGF:
-
Nerve Growth Factor
- PA:
-
Plasminogen Activator
- PAI:
-
Plasminogen Activator Inhibitor
- PAI-1:
-
Endothelial-type (type-1) Plasminogen Activator Inhibitor
- PAI-2:
-
Placental-type (type-2) Plasminogen Activator Inhibitor
- PDGF:
-
Platelet Derived Growth Factor
- TGFα:
-
Transforming Growth Factor-α
- TGFß:
-
Transforming Growth Factor-ß
- t-PA:
-
Tissue-type Plasminogen Activator
- u-PA:
-
Urokinase-type Plasminogen Activator
References
Andreasen PA, Nielsen LS, Kristensen P, Grøndahl-Hansen J, Skriver L and Danø K (1986) Plasminogen activator inhibitor from human fibrosarcoma cells binds urokinasetype plasminogen activator, but not its proenzyme. J. Biol. Chem. 261: 7644–7651.
Anzano MA, Roberts AB, Meyers CA, Komoriya A, Lamb LC, Smith JM and Sporn MB (1982) Synergistic interaction of two classes of transforming growth factors from murine sarcoma cells. Cancer Res. 42: 4776–4778.
Anzano MA, Roberts AB, Smith JM, Lamb LC and Sporn MB (1982) Purification by reverse-phase high-performance liquid chromatography of an epidermal growth factor-dependent transforming growth factor. Anal. Biochem. 125: 217–224.
Anzano MA, Roberts AB, Smith JM, Sporn MB and DeLarco JE (1983) Sarcoma growth factor from conditioned medium of virally transformed cells is composed of both type α and type-ß transforming growth factors. Proc. Natl. Acad. Sci. USA 80: 6264–6268.
Assoian RK, Grotendorst GR, Miller DM and Sporn MB (1984) Cellular transformation by coordinated action of three peptide growth factors from human platelets. Nature 309: 804–806.
Assoian RK, Komoriya A, Meyers CA, Miller DM and Sporn MB (1983) Transforming growth factor-ß in human platelets. Identification of a major storage site, purification, and characterization. J. Biol. Chem. 258: 7155–7160.
Cate RL, Mattaliano RJ, Hession C, Tizard R, Farber NM, Cheung A, Ninfa EG, Frey AZ, Gash DJ, Chow EB, Fisher RA, Bertonis JM, Torres G, Wallner BP, Ramachandran KL, Ragin RC, Manganaro TF, MacLaughlin DT and Donahoe PK (1986) Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell 45: 685–698.
Cheifetz S, Weatherbee JA, Tsang ML-S, Anderson JK, Mole JE, Lucas R and Massagué J (1987) The transforming growth factor-ß system, a complex pattern of cross-reactive ligands and receptors. Cell 48: 409–415.
Chen J-M and Chen W-T (1987) Fibronectin-degrading proteases from the membranes of transformed cells. Cell 48: 193–203.
Chiang C-P and Nilsen-Hamilton M (1986) Opposite and selective effects of epidermal growth factor and human platelet transforming growth factor-ß on the production of secreted proteins by murine 3T3 cells and human fibroblasts. J. Biol. Chem. 261: 10478–10481.
Childs CB, Proper JA, Tucker RF and Moses HL (1982) Serum contains a platelet-derived transforming growth factor. Proc. Natl. Acad. Sci. USA 79: 5312–5316.
Chua CC, Geiman DE, Keller GH and Ladda RL (1985) Induction of collagenase secretion in human fibroblast cultures by growth promoting factors. J. Biol. Chem. 260: 5213–5216.
Coffey RJJr., Kost LJ, Lyons RM, Moses HL and LaRusso NF (1987) Hepatic processing of transforming growth factor-ß in the rat. Uptake, metabolism, and biliary excretion. J. Clin. Invest. 80: 750–757.
Danø K, Andreasen PA, Grøndahl-Hansen J, Kristensen P, Nielsen LS and Skriver L (1985) Plasminogen activators, tissue degradation, and cancer. Adv. Cancer Res. 44: 139–266.
Davies RL, Rifkin DB, Tepper R, Miller A and Kucherlapati R (1983) A polypeptide secreted by transformed cells that modulates human plasminogen activator production. Science 221: 171–173.
de Martin R, Haendler B, Hofer-Warbinek R, Gaugitsch H, Schlüsener H, Seifert JM, Bodmer S, Fontana A and Hofer E (1987) Complementary DNA for human glioblastomaderived T cell suppressor factor, a novel member of the transforming growth factor-ß gene family. EMBO J. 6: 3676–3677.
DeLarco JE and Todaro GJ (1987) Growth factors from murine sarcoma virus-transformed cells. Proc. Natl. Acad. Sci. USA 75: 4001–4005.
Denhardt DT, Hamilton RT, Parfett CLJ, Edwards DR St. Pierre R, Waterhouse P and Nilsen-Hamilton M (1986) Close relationship of the major exreted protein of transformed murine fibroblasts to thiol-dependent cathepsins. Cancer Res. 46: 4590–4593.
Derynck R, Jarrett JA, Chen EY, Eaton DH, Bell JR, Assoian RK, Roberts AB, Sporn MB and Goeddel DV (1985) Human transforming growth factor-ß complementary DNA sequence and expression in normal and transformed cells. Nature 316: 701–705.
Derynck R, Jarrett JA, Chen EY and Goeddel DV (1986) The murine transforming growth factor fl precursor. J. Biol. Chem. 251: 4377–4379.
Derynck R, Goeddel DV, Ullrich A, Gutterman JU, Williams RD, Bringman TS and Berger WH (1987) Synthesis of messenger RNAs for transforming growth factors α and ß and the epidermal growth factor receptor by human tumors. Cancer Res. 47: 707–712.
Derynck R, Lindquist PB, Lee A, Wen D, Tamm J, Graycar JL, Rhee L, Mason AJ, Miller DA, Coffey RJ, Moses HL, and Chen EY (1988) A new type of transforming growth factor, TGF-ß3. EMBO J. 7: 3737–3743.
Eaton DL and Baker JB (1983) Phorbol esters and mitogens stimulate human fibroblast secretions of plasmin-activatable plasminogen activator and protease nexin, an antiactivator/antiplasmin. J. Cell Biol. 97: 323–328.
Edwards DR, Parfett CLJ and Denhardt DT (1985) Transcriptional regulation of two serum-induced RNAs in mouse fibroblasts: Equivalence of one species to 132 repetitive elements. Mol. Cell. Biol. 5: 3280–3288.
Edwards DR, Murphy G, Reynolds JJ, Whitham SE, Docherty AJP, Angel P, and Heath JK (1987) Transforming growth factor-ß modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. 6: 1899–1904.
Erickson LA, Ginsberg MH and Loskutoff DJ (1984) Detection and partial characterization of an inhibitor of plasminogen activator in human platelets. J. Clin. Invest. 74: 1465–1472.
Fairbairn S, Gilbert R, Ojakian G, Schwimmer R and Quigley JP (1965) The extracellular matrix of normal chick embryo fibroblasts: Its effect on transformed chick fibroblasts and its proteolytic degradation by the transformants. J. Cell Biol. 101: 1790–1796.
Florini JR, Roberts AB, Ewton DZ, Falen SL, Flanders KC and Sporn MB (1986) Transforming growth factor-ß: A very potent inhibitor of myoblast differentiation, identical to the differentiation inhibitor secreted by buffalo rat liver cells. J. Biol. Chem. 261: 16509–16513.
Frolik CA, Dart LL, Meyers CA, Smith DM and Sporn MB (1983) Purification and initial characterization of a type-ß transforming growth factor from human placenta. Proc. Natl. Acad. Sci. USA 80: 3676–3680.
Gentry LE, Webb NR, Lim GJ, Brunner AM, Ranchalis JE, Twardzik DR, Lioubin MN, Marquardt H and Purchio AF (1987) Type 1 transforming growth factor-ß: amplified expression and secretion of mature and precursor polypeptide in Chinese hamster ovary cells. Mol. Cell. Biol. 7: 4318–4327.
Gerwin BI, Keski-Oja J, Seddon M, Lechner JF and Harris CC (1989) Differential modulation by TGFß1 of the expression of urokinase and type-1 plasminogen activator inhibitor in normal and squamous differentiation-defective human bronchial epithelial cells. J. Cell. Physiol. (submitted).
Gold LI, Schwimmer R, Rostagno A and Quigley JP (1989) Human plasma fibronectin as a substrate for human urokinase. Biochem. J. (in press)
Gottesman MM (1978) Transformation-dependent secretion of a low molecular weight protein by murine fibroblasts. Proc. Natl. Acad. Sci. USA 75: 2767–2771.
Hanks SK, Armour R, Baldwin JH, Maldonado F, Spiess J and Holley RW (1988) Amino acid sequence of the BSC-1 cell growth inhibitor (polyergin) deduced from the nucleotide sequence of the cDNA. Proc. Natl. Acad. Sci. USA 85: 79–82.
Hekman CM and Loskutoff DJ (1985) Endothelial cells produce a latent inhibitor of plasminogen activators that can be activated by denaturants. J. Biol. Chem. 260: 11581–11587.
Holley RW, Armour R and Baldwin JH (1978) Density-dependent regulation of growth of BSC-1 cells in cell culture: Growth inhibitors formed by the cells. Proc. Natl. Acad. Sci. USA 75: 1864–1866.
Holley RW, Böhlen P, Fava R, Baldwin JH, Kleeman G and Armour R (1980) Purification of kidney epithelial cell growth inhibitors. Proc. Natl. Acad. Sc. USA 77: 5989–5992.
Huang JS, Huang SS and Deuel TF (1984) Specific covalent binding of platelet derived growth factor to human plasma α2-macroglobulin. Proc. Natl. Acad. Sci. USA 81: 342–346.
Huang SS, O'Grady P and Huang JS (1988) Human transforming growth 329-2 complex is a latent form of transforming growth factor-ß-α2-macroglobulin complex is a latent form of transforming growth factor-ß. J. Biol. Chem. 263: 1535–1541.
Hurme M, Sihvola M, Alitalo K and Keski-Oja J (1989) Transforming growth factor-ß does not alter interleukin-1 expression in cultured human macrophages. J. Cell. Biochem. 39: 467–475.
Ignotz RA and Massagué J (1985) Type-ß transforming growth factor controls the adipogenic differentiation of 3T3 fibroblasts. Proc. Natl. Acad. Sci. USA 82: 8530–8534.
Ignotz RA and Massagué J (1986) Transforming growth factor-ß stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J. Biol. Chem. 261: 4337–4345.
Jakowlew SBm Dillard PJ, Sporn MB and Roberts AB (1988) Complementary Deoxyribonucleic Acid Cloning of a Messenger Ribonucleic Acid Encoding Transforming Growth Factor-ß4 From Chicken Embryo Chondrocytes. Mol. Endocrinol. (in press)
Josso N, Picard J-Y and Tran D (1977) The antimüllerian hormone. Recent Prog. Horm. Res. 33: 117–158.
Keski-Oja J and Todaro GJ (1980) Specific effects of fibronectin-releasing peptides on the extracellular matrices of cultured human fibroblasts. Cancer Res. 40: 4722–4727.
Keski-Oja J and Vaheri A (1982) The cellular target for the plasminogen activator urokinase, in human fibroblasts-66.000 dalton protein. Biochim. Biophys. Acta 720: 141–146.
Keski-Oja J, Leof EB, Lyons RM, Coffey RJ and Moses HL (1987) Transforming growth factors and control of neoplastic cell growth. J. Cell. Biochem. 33: 95–107.
Keski-Oja J, Blasi F, Leof EB and Moses HL (1988) Regulation of the synthesis and activity of urokinase plasminogen activator in A549 human lung carcinoma cells by transforming growth factor-ß. J. Cell Biol. 106: 451–459.
Keski-Oja J, Postlethwaite AE and Moses HL (1988) Transforming growth factors in the regulation of malignant cell growth and invasion. Cancer Invest. 6: 705–724.
Keski-Oja J, Raghow R, Sawdey M, Loskutoff DJ, Postlethwaite AE, Kang AH and Moses HL (1988) Regulation of mRNAs for type-1 plasminogen activator inhibitor, fibronectin and type 1 procollagen by transforming growth factor-ß: Divergent responses in lung fibroblasts and carcinoma cells. J. Biol. Chem. 263: 3111–31115.
Keski-Oja J, Laiho M and Lohi J (1989) Activation of latent cell-derived transforming growth factor-ß by the plasminogen activator urokinase. J. Cell. Biol. 107, (pt. 3): 50a.
Keski-Oja J and Vartio T (1989) Pericellular substrate for urokinase is a gelatin degrading enzyme that is enhanced by transforming growth factor-ß. J. Cell. Biol. 109 (pt. 2). (in press)
Kimelman D and Kirschner M (1987) Synergistic induction of mesoderm by FGF and TGFß and the identification of an mRNA coding for FGF in the early Xenopus embryo. Cell 51: 869–877.
Knudsen BS, Harpel PC and Nachman RL (1987) Plasminogen activator inhibitor is associated with the extracellular matrix of cultured bovine smooth muscle cells. J. Clin. Invest. 80: 1082–1089.
Kooistra T, Sprengers ED and van Hinsbergh VWM (1986) Rapid inactivation of the plasminogen activator inhibitor upon secretion from cultured human endothelial cells. Biochem. J. 239: 497–503.
Kryceve-Martinerie C, Lawrence DA, Crochet J, Jullien P and Vigier P (1985) Further study of ß-TGFs released by virally transformed and non-transformed cells. Int. J. Cancer. 35: 553–558.
Laiho M, Saksela O and Keski-Oja J (1986) Transforming growth factor-ß alters plasminogen activator activity in human skin fibroblasts. Exp. Cell Res. 164: 399–407.
Laiho M, Saksela O, Andreasen PA and Keski-Oja J (1986) Enhanced production and extracellular deposition of the endothelial-type plasminogen activator inhibitor in cultured human lung fibroblasts by transforming growth factor-ß. J. Cell Biol. 103: 2403–2410.
Laiho M, Saksela O and Keski-Oja J (1987) Transforming growth factor-ß induction of type-1 plasminogen activator inhibitor. Pericellular deposition and sensitivity to exogenous urokinase. J. Biol. Chem. 262: 17467–17474.
Laiho M (1988) Modulation of extracellular proteolytic activity and anchorage-independent growth of cultured cells by sarcoma cell-derived factors. Relationships to transforming growth factor-ß. Exp. Cell Res. 176: 297–308.
Laiho M (1988) Transforming growth factor-ß in the regulation of extracellular proteolysis of cultured cells. Thesis. University of Helsinki, Helsinki, Finland.
Laiho M and Keski-Oja J (1989) Growth factors in the regulation of extracellular proteolysis: A review. Cancer Res. 49: 2533–2553.
Lawrence DA, Pircher R, Kryceve-Martinerie C and Jullien P (1984) Normal embryo fibroblasts release transforming growth factors in a latent form. J. Cell. Physiol. 121: 184–188.
Lawrence DA, Pircher R and Jullien P (1985) Conversion of a high molecular weight latent ß-TGF from chicken embryo fibroblasts into a low molecular weight active ß-TGF under acidic conditions. Biochem. Biophys. Res. Commun. 133: 1026–1034.
Levin EG (1983) Latent tissue plasminogen activator produced by human endothelial cells in culture: Evidence for an enzyme-inhibitor complex. Proc. Natl. Acad. Sci. USA 80: 6804–6808.
Levin EG (1986) Quantitation and properties of the active and latent plasminogen activator inhibitors in cultures of human endothelial cells. Blood 67: 1309–1313.
Levin EG and Santell L (1987) Association of a plasminogen activator inhibitor (PAI-1) with the growth substratum and membrane of human endothelial cells. J. Cell Biol. 105: 2543–2549.
Liotta LA, Tryggyason K, Garbisa S, Hart I, Foltz CM and Shafie S (1980) Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature 284: 67–68.
Lohi J, Pertovaara L, Sistonen L, Alitalo K and Keski-Oja J (1989) Regulation by TGFß of genes involved in growth control: interleukin-6. Ann. N.Y. Acad. Sci., submitted.
Lund LR, Riccio A, Andreasen PA, Nielsen LS, Kristensen P, Laiho M, Saksela O, Blasi F and Danø K (1987) Transforming growth factor-ß is a strong and fast acting positive regulator of the level of type-1 plasminogen activator inhibitor mRNA in WI-38 human lung fibroblasts. EMBO J. 6: 1281–1286.
Lyons RM, Gentry LE, Purchio AF and Moses HL (1989) Proteolytic processing and activation of latent transforming growth factor-ß. J. Cell. Biol. 107, (pt. 3): 51 a.
Lyons RM, Keski-Oja J and Moses HL (1988) Proteolytic activation of latent transforming growth factor-ß from fibroblast conditioned medium. J. Cell. Biol. 106: 1659–1665.
Machida CM, Muldoon LL, Rodland KD and Magun BE (1988) Transcriptional modulation of transin gene expression by epidermal growth factor and transforming growth factor-ß. Mol. Cell. Biol. 8: 2479–2483.
Madisen L, Webb T, Rose TM, Marquardt H, Ikeda T, Twardzik D, Seyedin S and Purchio AF (1988) Transforming growth factor-ß2: cDNA cloning and sequence analysis. DNA 7: 1–8.
Marquardt H, Lioubin MN and Ikeda T (1987) Complete amino acid sequence of human transforming growth factor type ß2. J. Biol. Chem. 262: 12127–12131.
Mason AJ, Hayflick JS, Ling N, Esch F, Ueno N, Ying S-Y, Guillemin R, Niall H and Seeburg PH (1985) Complementary DNA sequences of ovarian follicular fluid inhibin show precursor structure and homology with transforming growth factor-ß. Nature 318: 659–663
Massagué J, Cheifetz S, Endo T and Nadal-Ginard B (1986) Type-ß transforming growth factor is an inhibitor of myogenic differentiation. Proc. Natl. Acad. Sci. USA 83: 8206–8210.
Massagué J (1987) The TGF-ß family of growth and differentiation factors. Cell 49: 437–438.
Masui T, Wakefield LM, Lechner JF, La Veck MA, Sporn MB and Harris CC (1986) Type-ß transforming growth factor is the primary differentiation-inducing serum factor for normal human bronchial epithelial cells. Proc. Natl. Acad. Sci. USA 83: 2438–2442.
Matrisian LM, Glaichenhaus N, Gesnel M-C and Breathnach R (1985) Epidermal growth factor and oncogenes induce transcription of the same cellular mRNA in rat fibroblasts. EMBO J. 4: 1435–1440.
Matrisian LM, Leroy P, Ruhlmann C, Gesnel M-C and Breathnach R (1986) Isolation of the oncogene and epidermal growth factor-induced transin gene: complex control in rat fibroblasts. Mol. Cell. Biol. 6: 1679–1686.
Mignatti P, Robbins E and Rifkin DB (1986) Tumor invasion through the human amniotic membrane: Requirement for a proteinase cascade. Cell 47: 487–498.
Mimuro J, Schleef RR and Loskutoff DJ (1987) Extracellular matrix of cultured bovine aortic endothelial cells contains functionally active type 1 plasminogen activator inhibitor. Blood 70: 721–728.
Miyazono K, Hellman U, Wernstedt C and Heldin C-H (1988) Latent high molecular weight complex of transforming growth factor-ß1. Purification from human platelets and structural characterization. J. Biol. Chem. 263: 6407–6415.
Miyazono K and Heldin CH (1989) Role for carbohydrate structures in TGF-ß1 latency Nature 338: 158–160.
Moses HL, Branum EB, Proper JA and Robinson RA (1981) Transforming growth factor production by chemically transformed cells. Cancer Res. 41: 2842–2848.
Moses HL, Tucker RF, Leof EB, Coffey RJJ, Halper J and Shipley GD (1985) Type-ß transforming growth factor is a growth stimulator and a growth inhibitor. In: ‘Cancer Cells’ (eds. J. Feramisco, B. Ozanne and C. Stiles) Cold Spring Harbor Press, New York, vol. 3, pp. 65–75.
Mullins DE and Rorlich ST (1983) The role of proteinases in cellular invasiveness. Biochim. Biophys. Acta 695: 177–214.
Newman MJ, Lane EA, Nugent MA and Racker E (1986) Induction of anchorage-independent growth by epidermal growth factor and altered sensitivity to type-ß transforming growth factor in partially transformed rat kidney cells. Cancer Res. 46: 5842–5850.
Newman M, Lane EA, Iannotti A, Nugent MA, Pepinsky RB and Keski-Oja J (1989) Characterization and purification of a plasminogen activator inhibitor induced in normal rat kidney cells by transforming growth factor-ß. J. Biol. Chem., submitted.
Nilsen-Hamilton M and Holley RW (1983) Rapid selective effects by a growth inhibitor and epidermal factor on the incorporation of [35S]methionine into proteins secreted by African green monkey (BSC-1) cells. Proc. Natl. Acad. Sc. USA 80: 5636–5640.
Nugent MA, Lane EA, Keski-Oja J, Moses HL and Newman MJ (1989) Growth stimulation, altered regulation of high affinity epidermal growth factor receptors, and autocrine transformation in spontaneously transformed normal rat kidney cells by transforming growth factor-ß. Cancer Res. 49: 3884–3890.
O'Connor-McCourt MD and Wakefield LM (1987) Latent transforming growth factor-ß in serum: A specific complex with α2-macroglobulin. J. Biol. Chem. 262: 14090–14099.
O'Grady RL, Upfold LI and Stephens RW (1981) Rat mammary carcinoma cells secrete active collagenase and activate latent enzyme in the stroma via plasminogen activator. Int. J. Cancer 28: 509–515.
Ohta M, Greenberg JS, Anklesaria P, Bassols A and Massagué J (1987) Two forms of transforming growth factor-ß distinguished by multipotential haematopoietic progenitor cells. Nature 329: 539–541.
Ossowski L and Reich E (1983) Antibodies to plasminogen activator inhibit human tumor metastasis. Cell 35: 611–619.
Overall CM, Wrana JL and Sodek J (1989) Independent regulation of collagenase, 72-kDa progelatinase, and metalloendoproteinase inhibitor expression in human fibroblasts by transforming growth factor-\. J. Biol. Chem. 264: 1860–1869.
Padgett RW, St. Johnston RD and Gelbart WM (1987) A transcript from a Drosophila pattern gene predicts a protein homologous to the transforming growth factor-\ family. Nature 325: 81–84.
Pepinsky RB, Sinclair LK, Chow EP, Mattaliano RJ, Manganaro TF, Donahoe PK and Cate RL (1988) Proteolytic processing of Müllerian inhibiting substance produces a transforming growth factor-\ like fragment. J. Biol. Chem. 263: 18961–18964.
Pertovaara L, Sistonen L, Bos TJ, Vogt PK, Keski-Oja J and Alitalo K (1989) Enhanced jun gene expression is an early genomic response to transforming growth factor-\ stimulation. Mol. Cell. Biol. 9: 1255–1262.
Piersbacher MD and Ruoslahti E (1984) Variants of the cell recognition site of fibronectin that retain attachment promoting activity. Proc. Natl. Acad. Sci. USA 81: 5985–5988.
Pircher R, Jullien P and Lawrence DA (1986) \-transforming growth factor is stored in human blood platelets as a latent high molecular weight complex. Biochem. Biophys. Res. Commun. 136: 30–37.
Postlethwaite AE, Keski-Oja J, Moses HL and Kang AH (1987) Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor-\. J. Exp. Med. 165: 251–256.
Quigley JP (1976) Association of a protease (plasminogen activator) with a specific membrane fraction isolated from transformed cells. J. Cell Biol. 71: 472–486.
Quigley JP, Goldfarb RH, Scheiner C, O'Donnel-Tormey J and Yeo TK (1980) Plasminogen activator and the membrane of transformed cells. In: ‘Tumor cell surfaces and malignancy’ (eds. R.O. Hynes, and C.F. Fox), Progress in Clinical and Biological Research, Alan R. Liss Inc., New York, vol. 41, pp. 773–795.
Quigley JP, Gold LI, Schwimmer R and Sullivan L (1987) Limited cleavage of cellular fibronectin by plasminogen activator purified from transformed cells. Proc. Natl. Acad. Sci. USA 84: 2776–2780.
Ricco A, Lund LR, Sartorio R, Lania A, Andreasen PA, Dan∅ K and Blasi F (1988) The regulatory region of the human plaminogen activator inhibitor type-1 (PAI-1) gene. Nucl. Acid Res. 16: 2805–2824.
Rizzino A (1988) Transforming Growth Factor-\: Multiple effects on cell differentiation and extracellular matrices. Dev. Biol. 130: 411–422.
Roberts AB, Anzano MA, Lamb LC, Smith JM and Sporn MB (1981) New class of transforming growth factors potentiated by epidermal growth factor: Isolation from non-neoplastic tissues. Proc. Natl. Acad. Sci. USA 78: 5339–5343.
Roberts AB, Anzano MA, Lamb LC, Smith JM, Frolik CA, Marquardt H, Todaro GJ and Sporn MB (1982) Isolation from murine sarcoma cells of a new class of transforming growth factors potentiated by epidermal growth factor. Nature 295: 417–419.
Roberts AB, Anzano MA, Meyers CA, Wideman J, Blacher R, Pan Y-CE, Stein S, Lehrman SR, Smith LC, Lamb LC and Sporn MB (1983) Purification and properties of a type-\ transforming growth factor from bovine kidney. Biochemistry 22: 5692–5698.
Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl JH and Fauci AS (1986) Transforming growth factor type-\: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc. Natl. Acad. Sci. USA 83: 4167–4171.
Ronne H, Anundi H, Rask L and Peterson PA (1979) Nerve growth factor binds to serum alpha2-macroglobulin. Biochem. Biophys. Res. Commun. 87: 330–336.
Rosa F, Roberts AB, Danielpour D, Dart LL Sporn MB and Dawid IB (1988) Mesoderm induction in amphibians: The role of TGF\2-like factors. Science 239: 783–785.
Rossi P, Karsenty G, Roberts AB, Roche NS, Sporn MB and de Crombrugghe B (1988) A nuclear factor 1 binding site mediates the reanscriptional activation of a type 1 collagen promoter by transforming growth factor\. Cell 52: 405–414.
Saksela O, Laiho M and Keski-Oja J (1985) Regulation of plasminogen activator activity in human fibroblastic cells by fibrosarcoma cell-derived factors. Cancer Res. 45: 2314–2319.
Saksela O, Moscatelli D and Rifkin DB (1987) The opposing effects of basic fibroblast growth factor and transforming growth factor-\ on the regulation of plasminogen activator activity in capillary endothelial cells. J. Cell Biol. 105: 957–963.
Saksela O (1985) Plasminogen activation and regulation of pericellular proteolysis. Biochim. Biophys. Acta 823: 35–65.
Salo T, Liotta LA, Keski-Oja J, Turpeenniemi-Hujannen T and Tryggvason K (1982) Secretion of basement membrane collagen degrading enzyme and plasminogen activator by transformed cells-role in metastasis. Int. J. Cancer 30: 669–673.
Scher W (1987) The role of extracellular proteases in cell proliferation and differentiation. Lab. Invest. 57: 607–633.
Seyedin SM, Thomas TC, Thompson AY, Rosen DM and Piez KA (1985) Purification and characterization of two cartilage-inducing factors from bovine demineralized bone. Proc. Natl. Acad. Sci. USA 82: 2267–2271.
Seyedin SM, Thompson AY, Bentz H, Rosen DM, McPherson JM, Conti A, Siegel NR, Galluppi GR and Piez KA (1986) Cartilage-inducing factor-A: Apparent identity to transforming growth factor-\. J. Biol. Chem. 261: 5693–5695.
Seyedin SM, Segarini PR, Rosen DM, Thompson AY, Bentz H and Graycar J (1987) Cartilage-inducing factor-B is a unique protein structurally and functionally related to transforming growth factor\. J. Biol. Chem. 262: 1946–1949.
Sporn MB, Roberts AB, Shull JH, Smith JM, Ward JM and Sodek J (1983) Polypeptide transforming growth factors isolated from bovine sources and used for wound healing in vivo. Science 219: 1329–1331.
Sprengers ED and Kluft C (1987) Plasminogen activator inhibitors. Blood 69: 381–387.
Stoppelli MP, Tacchetti C, Cubellis MV, Corti A, Hearing VJ, Cassani G, Appella E and Blasi F (1986) Autocrine saturation of pro-urokinase receptors on human A431 cells. Cell 45: 675–684.
ten Dijke P, Hansen P, Iwata KK, Pieler C and Foulkes JG (1988) Identification of another member of the transforming growth factor type-\ gene family. Proc. Natl. Acad. Sci. USA 85: 4715–4719.
Thalacker FW and Nilsen-Hamilton M (1987) Specific induction of secreted proteins by transforming growth factor-\ and 12-0-tetradecanoylphorbol-13-acetate. J. Biol. Chem. 262: 2283–2290.
Thompson NL, Flanders KC, Smith JM, Ellingsworth LR, Roberts AB and Sporn MB (1989) Expression of transforming growth factor-\1 in specific cells and tissues of adult and neonatal mice. J. Cell Biol 108: 661–670.
Todaro GJ, DeLarco JE and Cohen S (1976) Transformation by murine and feline sarcoma viruses specifically blocks binding of epidermal growth factor to cells. Nature 264: 26–31.
Tucker RF, Shipley GD, Moses HL and Holley RW (1984) Growth inhibitor from BSC-1 cells is closely related to the platelet type-\ transforming growth factor. Science 226: 705–707.
Van Obberghen-Schilling E, Roche NS, Flanders KC, Sporn MB and Roberts AB (1988) Transforming growth factor-\1 positively regulates its own expression in normal and transformed cells. J. Biol. Chem. 263: 7741–7746.
Wahl SH, Hunt DA, Wakefield LM, McCartney-Francis N, Wahl LM, Roberts AB and Sporn MB (1987) Transforming growth factor type-\ induces monocyte chemotaxis and growth factor production. Proc. Natl. Acad. Sci. USA 84: 5788–5792.
Wakefield LM, Smith DM, Masui T, Harris CC, and Sporn MB (1987) Distribution and modulation of the cellular receptor for transforming growth factor-\. J. Cell Biol. 105: 965–975.
Weeks DL and Melton DA (1987) A maternal mRNA localized to the vegetal hemisphere in Xenopus eggs codes for a growth factor related to TGF\. Cell 51: 861–867.
Werb Z, Mainardi CL, Vater CA and Harris ED (1977) Endogenous activation of latent collagenase by rheumatoid synovial cells. N. Engl. J. Med. 296: 1017–1023.
Wrann M, Bodmer S, de Martin R, Siepl C, Hofer-Warbinek R, Frei K, Hofer E and Fontana A (1987) T-cell suppressor factor from human glioblastoma cells is a 12.5-kd protein closely related to transforming growth factor-\. EMBO J. 6: 1633–1636.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Keski-Oja, J., Lohi, J. & Laiho, M. Transforming growth factor-ßs as modulators of pericellular proteolytic events. Cytotechnology 2, 317–332 (1989). https://doi.org/10.1007/BF00364996
Issue Date:
DOI: https://doi.org/10.1007/BF00364996