Abstract
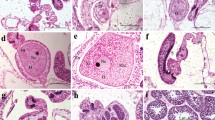
The reproductive ecology of Pavona gigantea Verrill and Gardineroseris planulata (Dana) was investigated in the equatorial eastern Pacific region from 1985 to 1994. These zooxanthellate scleractinian corals were adversely affected in this region during the 1982–1983 El Niño warming event. Both species were hermaphroditic, with individual colonies showing sequential cosexual development, thus resulting in dominantly outbreeding reproduction. Sexuality was mixed, with high percentages of gonochoric and hermaphroditic colonies in both species. Approximately 1:1 male-to-female gonad ratios were found in gonochoric and hermaphroditic colonies combined. Broadcast spawning was observed in P. gigantea in the Galápagos Islands, and the sudden disappearance of mature gametes and the presence of spent gonads suggest that G. planulata is also a broadcast spawner. Colonies of both species with ≲200 cm2 (10 cm diam) live tissue were nonreproductive. Estimated ages of the youngest reproductive colonies were 11 yr for P. gigantea and 20 yr for G. planulata. The percentage of all colonies of P. gigantea with gonads at nonupwelling sites (Caño Istand, Costa Rica and Uva Island, Panamá) ranged from 37 to 47%, respectively; colonies with gonads from upwelling environments (Saboga and Taboga Islands, Panamá) ranged from 31 to 39%, respectively, and reproductively active colonies from the thermally variable Galápagos islands comprised 40% of the collections. Compared with P. gigantea, the numbers of sexually active G. planulata colonies were roughly onehalf at nonupwelling Caño Island (20%) and Uva Island (25%) sites, or less (10%) at the upwelling Saboga Island site. Peak reproductive activity in P. gigantea occurred during the rainy season at all study sites. In the nonupwelling Costa Rican (Caño Island) and Panamainan (Uva Island) sites, mean monthly sea-surface temperatures (SSTs) were high (28 to 29°C), but slightly lower than in the dry season (29°C). In the upwelling Gulf of Panamá (Saboga and Taboga Islands), reproduction occurred after mean monthly SSTs increased from 24 to 28–29°C. In the Galápagos Islands, reproductive activity peaked during sea warming, when mean monthly SSTs reached 25°C. Sexually active colonies of G. planulata, present only at the main collection sites of Caño and Uva Islands, were also observed during the wet season. The presence of mature or spawned gonalds in both species mostly around new and full lunar phases suggests that spawning is at least weakly synchronized with moon phase. Fecundity estimates disclosed the following nonsignificant differences between sites for P. gigantea, expressed as egg production cm-2 colony surface surface yr-1: Galápagos (10 300 to 30 800), Uva Island (4900 to 9800), Caño Island (1800 to 7400), Saboga Island (600 to 1300) Taboga Island (1200 to 2400). Fecundity estimates for G. planulata were considerably lower: Uva Island (700 to 1400), Caño Island (500 to 1000). The sexual recruitment of P. gigantea into El Niño-Southern Oscillation (ENSO) 1982–1983-disturbed, equatorial eastern Pacific coral communities has been low, with only moderate recovery evident since 1983. G. planulata has revealed no sexual recruitment where seed populations are absent or rare (Caño Island, Galápagos Islands), and only low recruitment (Panamá) in areas with colonies that survived the ENSO disturbance.
Similar content being viewed by others
References
Babcock RC (1991) Comparative demography of three species of scleractinian corals using age-and size-dependent classifications. Ecol Monogr 61: 225–244
Babcock RC, Bull GD, Harrison PL, Heyward AJ, Oliver JK, Wallace CC, Willis BL (1986) Synchronous spawnings of 105 scleractinian coral species on the Great Barrier Reef. Mar Biol 90: 379–394
Birkeland C (1977) The importance of rate of biomass accumulation in early successional stages of benthic communities to the survival of coral recruits. Proc 3rd int coral Reef Symp 1: 15–21 [Taylor DL (ed) Rosenstiel School of Marine and Atmospheric Science, University of Miami, Miami, Florida]
Birkeland C, Rowley D, Randall RH (1981) Coral recruitment patterns at Guam. Proc 4th int coral Reef Symp 2: 339–344 [Gomez ED et al. (eds) Marine Sciences Center, University of the Philippines, Quezon City, Philippines]
Chornesky EA, Peters EC (1987) Sexual reproduction and colony growth in the scleractinian coral Porites astreoides. Biol Bull mar biol Lab, Woods Hole 172: 161–177
Connell JH, Keough MJ (1985) Disturbance and patch dynamics of subtidal marine animals on hard substrata. In: Pickett STA, White PS (eds) The ecology of natural disturbance and patch dynamics. Academic Press, Orlando, pp 125–151
Cortés J, Murillo MM, Guzmán HM, Acuña J (1984) Pérdida de zooxantelas y muerte de corales y otros organismos arrecifales en el Caribe y Pacífico de Costa Rica. Revta Biol trop 32: 227–231
Dai CF, Soong K, Fan TY (1993) Sexual reproduction of corals in northern and southern Taiwan. Proc 7th int coral Reef Symp 1: 448–455 [Richmond RH (ed) University of Guam Press, Mangilao, Guam]
Enfield DB, Calienes R, Pizarro L (1992) Oceanographic conditions along the coast of South America during the 1991–1992 El Niño/Southern Oscillation. Trop Oceans glob Atoms (TOGA) Notes 8: 1–4
Fadlallah YH (1993) Sexual reproduction development and larval biology in scleractinian oorals. A review. Coral Reefs 2: 129–150
Fan TY, Dai CF (1993) Reproductive cycles of Echinophyllia aspera in southern and northern Taiwan. Proc 7th int coral Reef Symp 1: p. 501 (Abstract) [Richmond RH (ed) University of Guam Press, Mangilao, Guam]
Glynn PW (1985) El Niño-associated disturbance to coral reefs and post disturbance mortality by Acanthaster planci. Mar Ecol Prog Ser 26: 295–300
Glynn PW (1988) El Niño warming, coral mortality and reef framework destruction by echinoid bioerosion in the eastern Pacific. Galaxea 7: 129–160
Glynn PW (1990) Coral mortality and disturbances to coral reefs in the tropical eastern Pacific. In: Glynn PW (ed) Global ecological consequences of the 1982–1983 El Niño-Southern Oscillation. Elsevier, Amsterdam, pp 55–126 (Elsevier Oceanogr Ser)
Glynn PW (1994) State of coral reefs in the Galápagos Islands: natural versus anthropogenic impacts. Mar Pollut Bull 29: 131–140
Glynn PW, Colley SB, Eakin CM, Smith DB, Cortés J, Gassman NJ, Guzmán HM, Del Rosario JB, Feingold JS (1994) Reef coral reproduction in the eastern Pacific: Costa Rica, Panamá, and Galápagos Islands (Ecuador). II. Poritidae. Mar Biol 118: 191–208
Glynn PW, D'Croz L (1990) Experimental evidence for high temperature stress as the cause of El Niño-coincident coral mortality. Coral Reefs 8: 181–191
Glynn PW, Feingold JS (1992) Hydrocoral species not extinct. Science, Wash 257: 1845
Glynn PW, Gassman NJ, Eakin CM, Cortés J, Smith DB, Guzmán HM (1991) Reef coral reproduction in the eastern Pacific: Costa Rica, Panamá, and Galápagos Islands (Ecuador). I. Pocilloporidae. Mar Biol 109: 355–368
Glynn PW, Imai R, Sakai K, Nakano Y, Yamazato K (1993) Experimental responses of Okinawan (Ryukyu Islands Japan) reef corals to high sea temperature and UV radiation. Proc 7th int coral Reef Symp 1: 27–37 [Richmond RH (ed) University of Guam Press, Mangilao, Guam]
Glynn PW, Weerdt WH de (1991) Elimination of two reef-building hydrocorals following the 1982–83 El Niño warming event. Science, Wash 253: 69–71
Glynn PW, Wellington GM (1983) Corals and coral reefs of the Galápagos Islands. University of California Press, Berkeley
Guzmán HM, Cortés J (1989) Coral reef community structure at Caño Island, Pacific Costa Rica. Pubbl Staz zool Napoli (I: Mar Ecol) 10: 21–43
Guzmán HM, Cortés J, Richmond RH, Glynn PW (1987) Electos del fenómeno de El Niño Oscilación Sureña 1982/83 en los arrecifes coralinos de la Isla del Caño, Costa Rica. Revta Biol trop 35: 325–332
Harriott VJ (1992) Recruitment patterns of scleractinian corals in an isolated sub-tropical reef system. Coral Reefs 11: 215–219
Harrison PL, Wallace CC (1990) Reproduction, dispersal and recruitment of scleractinian corals. In: Dubinsky Z (ed) Coral reefs, ecosystems of the world. 25. Elsevier, Amsterdam, pp 133–207
Heyward AJ, Babcock RC (1986) Self- and cross-fertilization in scleractinian corals. Mar Biol 90: 191–195
Heyward AJ, Yamazato K, Yeemin T, Minei M (1987) Sexual reproduction of corals in Okinawa. Galaxea 6: 331–343
Highsmith R (1982) Reproduction by fragmentation in corals. Mar Ecol Prog Ser 7: 207–226
Jokiel PL, Coles SL (1990) Response of Hawaiian and other Indo-Pacific reef corals to elevated temperature. Coral Reefs 8: 155–162
Kojis BL (1986) Sexual reproduction in Acropora (Isopora) (Coelenterata: Scleractinia). II. Latitudinal variation in A. palifera from the Great Barrier Reef and Papua New Guinea. Mar Biol 91: 311–318
Kojis BL, Quinn NJ (1981) Aspects of sexual reproduction and larval development in the shallow water hermatypic coral Goniastrea australensis (Edwards and Haime, 1857). Bull mar Sci 31: 558–573
Kojis BL, Quinn NJ (1984) Seasonal and depth variation in fecundity of Acropora palifera at two reefs in Papua New Guinea. Coral Reefs 3: 165–172
Kojis BL, Quinn NJ (1994) Biological limits to Caribbean reef recovery, a comparison with western South Pacific reefs. In Ginsburg RN (compiler) Proceedings of the Colloquium of Global Aspects of Coral Reefs: Health, Hazards and History, 1993. Rosenstiel School of Marine and Atmospheric Science, University of Miami, pp 353–359
Luna LG (1968) Manual of histologic staining methods. McGraw-Hill, New York
Maragos JE (1974) Reef corals of Fanning Island. Pacif Sci 28: 247–255
Oliver JK, Babcock RC, Harrison PL, Willis BL (1989) Geographic extent of mass coral spawning: clues to ultimate causal factors. Proc 6th int coral Reef Symp 2: 803–810 [Choat JH et al. (eds) Sixth International Coral Reef Symposium Executive Committee, Townsville, Australia]
Pearson RG (1981) Recovery and recolonization of coral reefs. Mar Ecol Prog Ser 4: 105–122
Policansky D (1982) Sex change in plants and animals. A Rev Ecol Syst 13: 471–495
Prahl H von (1985) Blanqueo masivo y muerte de corales hermatípicos en el Pacífico Colombiano atribuídos al fenómeno de El Niño 1982–83. Boln ERFEN (CPPS, Bogotá) 12: 22–24
Quinn WH (1993) The large-scale ENSO event, the El Niño and other important regional features. Bull Inst fr Étud and 22: 13–34
Reyes Bonilla H (1993) Biogeografia y ecología de los corales hermatípicos (Anthozoa: Scleractinia) del Pacifico de Mexico. In: Salazar-Vallejo SI, González NE (eds) Biodiversidad marina y costera de Mexico. Comisión Nacional para el Conocimiento y Aprovechamiento de la Biodiversidad y Centro de Investigaciónes de Quintana Roo (CIQRO). Mexico, pp 207–222
Richmond RH (1985) Variations in the population biology of Pocillopora damicornis across the Pacific. Proc 5th int coral Reef Congr 6: 101–106 [Gabrie et al. (eds) Antenne Museum-EPHE, Moorea, French Polynesia]
Richmond RH (1987) Energetic relationships and biogeographical differences among fecundity, growth and reproduction in the reef coral Pocillopora damicornis. Bull mar Sci 41: 594–604
Richmond RH, Hunter CL (1990) Reproduction and recruitment of corals: comparisons among the Caribbean, the tropical Pacific, and the Red Sea. Mar Ecol Prog Ser 60: 185–203
Robinson G (1985) The influence of the 1982–83 El Niño on Galápagos marine life. In: Robinson G, del Pino EM (eds) El Niño in the Galápagos Islands: the 1982–1983 event. Charles Darwin Foundation for the Galápagos Islands, Quito, Ecuador, pp 153–190
Sammarco PW, Andrews JC (1988) Localized dispersal and recruitment in Great Barrier reef corals: the Helix experiment. Science, Wash 239: 1422–1424
Smith DB (1991) The reproduction and recruitment of Porites panamensis Verrill at Uva Island, Pacific Panamá. MS thesis. University of Miami, Florida
Squires DF (1959) Results of the Puritan-American Museum of Natural History Expedition to western Mexico. 7. Corals and coral reefs in the Gulf of California. Bull Am Mus nat Hist 118: 367–432
Stimson JS (1978) Mode and timing of reproduction in some common hermatypic corals of Hawaii and Enewetak. Mar Biol 48: 173–184
Stobart B, Babcock RC, Willis BL (1993) Biannual spawning of three species of scleractinian coral from the Great Barrier Reef. Proc 7th int coral Reef Symp 1: 494–499 [Richmond RH (ed) University of Guam Press, Mangilao, Guam]
Szmant AM, Gassman NJ (1990) The effects of prolonged “bleaching” on the tissue biomass and reproduction of the reef coral Montastrea annularis. Coral Reefs 8: 217–224
Tide Tables (1992) High and low water predictions, west coast of North and South America, including the Hawaiian Island. NOAA, US Department of Commerce, US Government Printing Office, Washington, DC
Van Moorsel GWNM (1983) Reproductive strategies in two closely related stony corals (Agaricia, Scleractinia). Mar Ecol Prog Ser 13: 273–283
Veron JEN (1993) A biogeographic database of hermatypic corals. Species of the central Indo-Pacific genera of the world. Monograph Ser Aust Inst mar Sci 10: 1–433
Wallace CC (1985) Seasonal peaks and annual fluctuations in recruitment of juvenile scleractinian corals. Mar Ecol Prog Ser 21: 289–298
Wellington GM (1982) Depth zonation of corals in the Gulf of Panama: control and facilitation by resident reef fishes. Ecol Monogr 52: 223–241
Wellington GM, Glynn PW (1983) Environmental influences on skeletal banding in eastern Pacific (Panamá) corals. Coral Reefs 1: 215–222
Willis BL, Babcock RC, Harrison PL, Oliver TK (1985) Patterns in the mass spawning of corals on the Great Barrier Reef from 1981 to 1984. Proc 5th int coral Reef Congr 4: 343–348 [Gabrié C et al. (eds) Antenne Museum-EPHE, Moorea, French Polynesia]
Author information
Authors and Affiliations
Additional information
Communicated by N. H. Marcus, Tallahassee
Rights and permissions
About this article
Cite this article
Glynn, P.W., Colley, S.B., Gassman, N.J. et al. Reef coral reproduction in the eastern Pacific: Costa Rica, Panamá, and Galápagos Islands (Ecuador). III. Agariciidae (Pavona gigantea and Gardineroseris planulata). Marine Biology 125, 579–601 (1996). https://doi.org/10.1007/BF00353270
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00353270