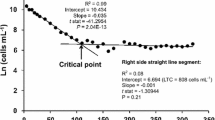
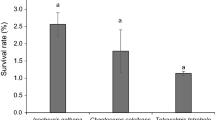
Abstract
I investigated selective particle ingestion by oyster larvae (Crassostrea virginica) feeding on natural seston from Chesapeake Bay and laboratory-cultured algae of different sizes or chemical content. In 15 of 16 experiments with complex natural suspensions as food, small(<150 μm) and large (>150 μm) larvae selected most strongly for small (2 to 4 μm) food particles, but in the presence of a large (>10 μm)-cell dinoflagellate bloom, large larvae strongly selected much larger (22 to 30 μm) food material (presumably dinoflagellates). When fed simplified mixtures of four cultured algal species (Synechococcus bacillaris, Isochrysis sp., Dunaliella tertiolecta, and Prorocentrum minimum) ranging in size from 1 to 11 μm, small larvae preferred 1 μm algae while large larvae preferred 11 μm algae. In experiments with algal mixtures, and with suspensions of natural particles and added algae, large larvae preferred algal species harvested from exponential-phase cultures over other species from stationary-phase cultures. Larval ingestion rates of the cultured alga Thalassiosira pseudonana were about three times higher for cells with a low carbon:nitrogen ratio (7.2:1) than for high C:N ratio (16.2:1) cells when these cells were offered separately in suspensions of equal concentration. As a result, more algal cells, algal C, and algal N was ingested by larvae fed low C:N cells. However, larvae did not show a significant preference for either type of cell when they were offered in a 1:1 cell mixture. Feeding patterns of C. virginica larvae in natural food suspensions can vary with the composition of these complex suspensions, and ingestion seems dependent not only on the size, but on the growth rate and chemical quality of food particles.
Similar content being viewed by others
References
Allan JD, Richman S, Heinle DR, Huff R (1977) Grazing in juvenile stages of some estuarine calanoid copepods. Mar Biol 43: 317–331
Appelmans N (1994) Sites of particle selection determined from observations of individual feeding larvae of the sand dollar Dendraster excentricus. Limnol Oceanogr 39: 404–411
Baldwin BS (1992) The natural diet and feeding behavior of planktotrophic larvae of the eastern oyster Crassostrea virginica (Gmelin). Doctoral dissertation. University of Maryland, College Park
Baldwin BS, Newell RIE (1991) Omnivorous feeding by planktotrophic larvae of the eastern oyster Crassostrea virginica. Mar Ecol Prog Ser 78: 285–301
Baldwin BS, Newell RIE (1995) Relative importance of different size food particles in the natural diet of oyster larvae (Crassostrea virginica). Mar Ecol Prog Ser 120: 135–145
Bautista B, Harris RP (1992) Copepod gut contents, ingestion rates and grazing impact on phytoplankton in relation to size structure of zooplankton and phytoplankton during a spring bloom. Mar Ecol Prog Ser 82: 41–50
Bayne BL (1983) The physiological ecology of marine molluscan larvae. In: Verdonk NH, van den Biggelaar JAM, Tompa A (eds) The Mollusca. Vol III. Development. Academic Press, New York, pp 229–343
Berggreen U, Hansen B, Kiørboe T (1988) Food size spectra, ingestion and growth of the copepod Acartia tonsa during development: implications for determination of copepod production. Mar Biol 99: 341–352
Bernard C, Rassoulzadegan F (1993) The role of picoplankton (cyanobacteria and plastidic picoflagellates) in the diet of tintinnids. J Plankton Res 15: 361–373
Butler NM, Suttle CA, Neill WE (1989) Discrimination by freshwater zooplankton between single algal cells differing in nutritional status. Oecologia 78: 368–372
Chesson, J (1978) Measuring preference in selective predation. Ecology 59: 211–215
Claustre H, Marty J-C, Cassiani L, Daguat J (1988) Fatty acid dynamics in phytoplankton and microzooplankton communities during a spring bloom in the coastal Ligurian Sea: ecological implications. Mar microb Fd Webs 3: 51–66
Confer JL, Moore MV (1987) Interpreting selectivity indices calculated from field data or conditions of prey replacement. Can J Fish aquat Sciences 44: 1529–1533
Cowles TJ, Olson RJ, Chisholm SW (1988) Food selection by copepods: discrimination on the basis of food quality. Mar Biol 100: 41–49
Davidson K, Flynn KJ, Cunningham A (1991) Relationships between photopigments, cell carbon, cell nitrogen and growth rate for a marine nanoflagellate. J exp mar Biol Ecol 153: 87–96
Enright CT, Newkirk GF, Cragie JS, Castell JD (1986) Growth of juvenile Ostrea edulis L. fed Chaetoceros gracilis Schütt of varied chemical composition. J exp mar Biol Ecol 96: 15–26
Flaak AR, Epifanio CE (1978) Dietary protein levels and growth of the oyster Crassostrea virginica. Mar Biol 45: 157–163
Fritz LW, Lutz RA, Foote MA, Van Dover CL, Ewart JW (1984) Selective feeding and grazing rates of oyster (Crassostrea virginica) larvae on natural phytoplankton assemblages. Estuaries 7: 513–518
Gallager SM (1988) Visual observations of particle manipulation during feeding in larvae of a bivalve mollusc. Bull mar Sci 43: 344–365
Gallegos CL (1992) Phytoplankton photosynthesis, productivity, and species composition in a eutrophic estuary: comparison of bloom and non-bloom assemblages. Mar Ecol Prog Ser 81: 257–267
Gifford DJ (1991) The protozoan-metazoan trophic link in pelagic ecosystems. J Protozool 38: 81–86
Goldman JC, McCarthy JJ, Peavey DW (1979) Growth rate influence on the chemical composition of phytoplankton in oceanic waters. Nature, Lond 279: 210–215
Guillard RR (1958) Some factors in the use of nanoplankton cultures as food for larval and juvenile bivalves. Proc natn Shellfish Ass 48: 134–142
Guillard RR (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH (eds) Culture of marine invertebrate animals. Plenum Press, New York, pp 29–60
Guillard RR, Wangersky PJ (1958) The production of extracellular carbohydrates by some marine flagellates. Limnol Oceanogr 3: 449–454
Hansen B (1991) Feeding behaviour in larvae of the opisthobranch Philine apertau. II. Food size spectra and particle selectivity in relation to larval behavior and morphology of the velar structures. Mar Biol 111: 263–270
Harrison PJ, Thompson PA, Calderwood GS (1990) Effects of nutrient and light limitation on the biochemical composition of phytoplankton. J appl Phycol 2: 45–56
Hart MW (1991) Particle captures and method of suspension feeding by echinoderm larvae. Biol Bull mar biol Lab, Woods Hole 180: 12–27
Hitchcock GL (1982) A comparative study of the size-dependent organic composition of marine diatoms and dinoflagellates. J Plankton Res 4: 363–377
Houde SEL, Roman MR (1987) Effects of food quality on the functional ingestion response of the copepod Acartia tonsa. Mar Ecol Prog Ser 40: 69–77
Huntley ME, Ciminiello P, Lopez MDG (1987) Importance of food quality in determining development and survival of Calanus pacificus (Copepoda: Calanoida). Mar Biol 95: 103–113
Huntley ME, Sykes P, Rohan S, Marin V (1986) Chemically-mediated rejection of dinoflagellate prey by the copepods Calanus pacificus and Paracalanus parvus: mechanism, occurrence and significance. Mar Ecol Prog Ser 28: 105–120
Ivlev VS (1961) Experimental ecology of the feeding of fishes. Yale University Press, New Haven, Connecticut
Jezequel VM, Poulet SA, Harris RP, Moal J, Samain JF (1988) Interspecific and intraspecific composition and variation of free amino acids in marine phytoplankton. Mar Ecol Prog Ser 44: 303–313
Kiørboe T, Andersen KP, Dam HG (1990) Coagulation efficiency and aggregate formation in marine phytoplankton. Mar Biol 107: 235–245
Kleppel GS, Holliday DV, Pieper RE (1991) Trophic interations between copepods and microplankton: a question about the role of diatoms. Limnol Oceanogr 36: 172–178
Langdon CJ, Waldcock MJ (1981) The effect of algal and artificial diets on the growth and fatty acid composition of Crassostrea gigas spat. J mar biol Ass UK 61: 431–448
Lechowicz MJ (1982) The sampling characteristics of electivity indices. Oecologia 52: 22–30
Lorenzen CJ (1966) A method for the continuous measurement of in vivo chlorophyll concentration. Deep-Sea Res 13: 223–227
Luckenbach MW, Sellner KG, Shumway SE, Greene K (1993) Effects of two bloom-forming dinoflagellates, Prorocentrum mariae-labouriae and Gyrodinium uncatenum, on growth and survival of the eastern oyster, Crassostrea virginica (Gmelin, 1791). J Shellfish Res 12: 411–416
Mackie G (1969) Quantitative studies of feeding in the oyster Crassostrea virginica. Proc natn Shellfish Ass 59: 6–7 (Abstract)
Malej A, Harris RP (1993) Inhibition of copepod grazing by diatom exudates: a factor in the development of mucus aggregates? Mar Ecol Prog Ser 96: 33–42
Marin V, Huntley ME, Frost B (1986) Measuring feeding rates of pelagic herbivores: analysis of experimental design and methods. Mar Biol 93: 49–58
Moal J, Martin-Jezequel V, Harris RP, Samain J-F, Poulet SA (1987) Interspecific and intraspecific variability of the chemical composition of marine phytoplankton. Oceanol Acta 10: 339–346
Morey-Gaines G (1979) The ecological role of red tides in the Los Angeles-Long Beach Harbor food web. In: Taylor DL, Seliger HH (eds) Toxic dinoflagellate blooms. Elsevier North Holland, Inc., New York, pp 315–320
Morris I (1981) Photosynthetic products, physiological state, and phytoplankton growth. Can Bull Fish aquat Sciences 210: 83–102
Post AF, Dubinsky Z, Wyman K, Falkowski PG (1985) Physiological responses of a marine planktonic diatom to transitions in growth irradiance. Mar Ecol Prog Ser 25: 141–149
Poulet SA, Marsot P (1978) Chemosensory grazing by marine calanoid copepods (Arthropoda: Crustacea). Science, NY 200: 1403–1405
Rassoulzadegan F, Fenaux L (1979) Grazing of echinoderm larvae (Paracentrotus lividus and Arbacia lixula) on natually occurring particulate matter. J Plankton Res 1: 215–223
Rassoulzadegan F, Fenaux L, Strathmann RR (1984) Effect of flavor and size on selection of food by suspension-feeding plutei. Limnol Oceanogr 29: 357–361
Richman S, Heinle DR, Huff R (1977) Grazing by adult estuarine calanoid copepods of the Chesapeake Bay. Mar Biol 42: 69–84
Riisgård HU (1988) Feeding rates in hard clam (Mercenaria mercenaria) veliger larvae as a function of algal (Isochrysis galbana) concentration. J Shellfish Res 7: 377–380
Riisgård HU, Randlov A, Kristensen PS (1980) Rates of water processing, oxygen consumption and efficiency of particle retention in veligers and young post-metamorphic Mytilus edulis. Ophelia 19: 37–47
Rivkin RB, Bosch I, Pearse JS, Lessard EJ (1986) Bacterivory: a novel feeding mode for asteroid larvae. Science, NY 223: 1311–1314
Scott JM (1980) Effect of growth rate of the food alga on the growth/ingestion efficiency of a marine herbivore. J mar biol Ass UK 60: 681–702
Seliger HH, Boggs JA, Rivkin RB, Biggley WH, Aspden KRH (1982) The transport of oyster larvae in an estuary. Mar Biol 71: 57–72
Sellner KG, Kachur ME (1987) Phytoplankton: relationships between phytoplankton, nutrients, oxygen flux and secondary producers. In: Heck KL, Jr. (ed) Ecological studies in the middle reach of Chesapeake Bay. Springer-Verlag, New York, pp 12–37
Shumway SE (1990a) A review of the effects of algal blooms on shellfish and aquaculture. J Wld Aquacult Soc 21: 65–104
Shumway SE (1990b) Auditing the impact of toxic algal blooms on oysters. Envir Auditor 2: 41–56
Sprung M (1984) Physiological energetics of mussel larvae (Mytilus edulis). II. Food uptake. Mar Ecol Prog Ser 17: 295–305
Steidinger KA (1979) Collection, enumeration, and identification of free-living marine dinoflagellates. In: Taylor DL, Seliger HH (eds) Toxic dinoflagellate blooms. Elsevier North Holland, Inc, New York, pp 319–331
Sterner RW, Hagemeir DD, Smith WL, Smith RF (1993) Phytoplankton nutrient limitation and food quality for Daphnia. Limnol Oceanogr 38: 857–871
Sterner RW, Smith RF (1993) Clearance, ingestion and release of N and P by Daphnia obtusa feeding on Scenedesmus acutus of varying quality. Bull mar Sci 53: 228–239
Strathmann RR (1987) Larval feeding. In: Giese AC, Pearse JS, Pearse VB (eds) Reproduction of marine invertebrates. Vol. IX. General aspects: seeking unity in diversity. Blackwell Scientific Publications, Palo Alto, California, pp 465–550
Thompson PA, Harrison PJ (1992) Effects of monospecific algal diets of varying biochemical composition on the growth and survival of Pacific oyster (Crassostrea gigas) larvae. Mar Biol 113: 645–654
Utting SD (1986) A preliminary study on the growth of Crassostrea gigas larvae and spat in relation to dietary protein. Aquaculture, Amsterdam 56: 123–138
Walne PR (1965) Observations on the influence of food supply and temperature on the feeding and growth of the larvae of Ostrea edulis. Fishery Invest, Lond 2: 24: 1–45
Vanderploeg HA, Scavia D (1979) Calculation and use of selectivity coefficients of feeding: zooplankton grazing. Ecol Modelling 7: 135–149
Van Valkenburg SD, Flemer DA (1974) The distribution and productivity of nannoplankton in a temperate estuarine area. Estur cstl mar Sci 2: 311–322
White AW (1981) Marine zooplankton can accumulate and retain dinoflagellate toxins and cause fish kills. Limnol Oceanogr 26: 103–109
Whyte JNC, Bourne N, Hodgson CA (1989) Influence of algal diets on biochemical composition and energy reserves in Patinopecten yessoensis (Jay) larvae. Aquaculture, Amsterdam 78: 333–347
Wikfors GH (1986) Altering growth and gross chemical composition of two microalgal molluscan food species by varying nitrate and phosphate. Aquaculture, Amsterdam 59: 1–14
Wilson JH (1979) Observations on the grazing rates and growth of Ostrea edulis L. larvae when fed algal cultures of different ages. J exp mar Biol Ecol 38: 187–199
Wilson JH (1980) Particle retention and selection by larvae and spat of Ostrea edulis in algal suspensions. Mar Biol 57: 135–145
Zubkoff PC, Munday JC, Rhodes RG, Warinner JE (1979) Mesoscale features of summer (1975–1977) dinoflagellate blooms in the York River, Virginia (Chesapeake Bay estuary). In: Taylor DL, Seliger HH (eds) Toxic dinoflagellate blooms. Elsevier North Holland, Inc, New York, pp 279–286
Author information
Authors and Affiliations
Additional information
Communicated by N.H. Marcus, Tallahassee
Rights and permissions
About this article
Cite this article
Baldwin, B.S. Selective particle ingestion by oyster larvae (Crassostrea virginica) feeding on natural seston and cultured algae. Marine Biology 123, 95–107 (1995). https://doi.org/10.1007/BF00350328
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00350328