Abstract
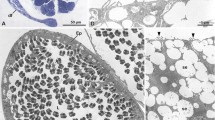
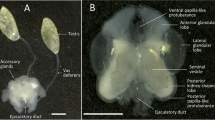
Assessing the possibility that external fertilization has ‘re-evolved’ requires the study of monophyletic groups that exhibit various reproductive methods. Maldanid polychaetes show a range of reproductive mechanisms, though previous studies of reproduction have hitherto been restricted to larger species with external fertilization. Micromaldane pamelae Rouse and M. nutricula Rouse are small, gonochoristic maldanids that brood directly developing larvae. Both species have sperm with elongate nuclei and an acrosome extending down each side of the anterior end of the nucleus. A true midpiece is absent; two mitochondria extend along the posterior region of the nucleus. Spermatids develop synchronously in large clusters connected by a cytophore. In M. pamelae sperm are released into the water as spermatozeugmata. These are comprised of clusters of sperm with their tails oriented to the centre and the sperm heads facing outwards. Females of M. pamelae and M. nutricula bear pairs of spermathecae ventrally (M. pamelae three pairs, between setigers 10 and 11, 11 and 12 and 12 and 13 and M. nutricula two pairs, between setigers 10 and 11 and setigers 11 and 12). The blind sacs are epidermal invaginations bound closely together. The entrance to each spermatheca may only be 1 to 2 μm across with each spermatheca holding several hundred sperm. This represents the first detailed study of spermathecae in the Capitellida. The occurrence and structure of spermathecae and spermatozeugmata in other groups are discussed and compared with Micromaldane spp. Comparisons are made with non-polychaetes with the purpose of discussing functional aspects of reproductive mechanisms in marine metazoans in general. Elongate sperm nuclei are associated with sperm storage and/or large egg size. The lack of an elongate sperm midpiece may be an indicator of having to swim in water but does not contraindicate sperm storage. Spermatozeugmata may serve as an indication of sperm storage and brooding of larvae. Speculations on the phylogenetic significance of these reproductive features are limited by the fact that supposedly modified (i.e., derived) states may reflect functional/structura, constraints of small body size.
Similar content being viewed by others
Literature cited
Adiyodi, K. G. (1985). Annelida. In: Adiyodi, K. G., Adiyodi, R. G. (eds.) Reproductive biology of invertebrates. III. Accessory sex glands. John Wiley & Sons, Chichester, p. 189–250
Ashworth, J. H. (1904). Memoir on Arenicola. The lugworm. Proc. Trans. Lpool biol. Soc. 18: 209–326
Bentley, M. G., Pacey, A. A. (1989). A scanning and electron microscopical study of sperm development and activation in Arenicola marina L. (Annelida: Polychaeta). Invert. Reprod. Dev. 15: 211–219
Bookout, C. G. (1949). The development of Axiothella mucosa (Andrews). J. Morph. 84: 145–183
Cazaux, C. (1972). Développment larvaire d'annélides polychétes (bassin d'Arcachon). Archs Zool. exp. gén. 113: 71–108
Clavier, J. (1983). Description du cycle biologique d'Euclymene oerstedi (Annelide Polychète) dans le bassin maritime de la Rance. C. r. hebd. Séanc. Acad. Sci., Paris 296: 483–486
Daddow, L. Y. M. (1983). A double lead stain method for enhancing contrast of ultrathin sections in electron microscopy: a modified multiple staining technique. J. Microscopie 129: 147–153
Downing, E. R. (1911). The formation of the spermatophore in Arenicola and a theory of the alternation of generations in animals. J. Morph. 22: 1001–1043
Duncan, A. (1960). The spawning of Arenicola marina (L.) in the British Isles. Proc. zool. Soc. Lond. 134: 137–156
Eckelbarger, K. J. (1974). Population biology and larval development of the terebellid polychaete Nicolea zostericola. Mar. Biol. 27: 101–113
Eckelbarger, K. J., Grassle, J. P. (1987). Spermatogenesis, sperm storage and comparative morphology in nine species of Capitella, Capitomastus and Capitellides (Polychaeta: Capitellidae). Mar. Biol. 95: 415–429
Eisig, H. D. (1887). Die Capitelliden des Golfes von Neapel. Fauna Flora Golf. Neapel 16: 1–906
Farke, H., Berghuis, E. M. (1979). Spawning, larval development and migration behaviour of Arenicola marina in the laboratory. Neth. J. Sea Res. 13: 512–528
Fauchald, K. (1977). The polychaete worms, definitions and keys to the orders, families and genera. Sci. Ser. Nat. Hist. Mus. Los Angeles County 28: 1–188
Franzén, Å. (1956). On spermiogenesis, morphology of the spermatozoon and biology of fertilization among invertebrates. Zool. Bidr. Upps. 31: 355–482
Franzén, Å., Sensenbaugh, T. (1984). Fine structure of spermiogenesis in the archiannelid Nerilla antennata Schmidt. Vidensk. Meddr dansk naturh. Foren. 145: 23–36
Fukomoto, M. (1981). The spermatozoa and spermiogenesis of Perophora formosana (Ascidia) with special reference to the striated apical structure and the filamentous structures in the mitochondrian. J. Ultrastruct. Res. 77: 37–53
Goodrich, E. S. (1930). A new hermaphrodite syllid. Q. Jl. microsc. Sci. 73: 651–666
Herpin, R. (1925). La ponte et la développement chez une Annélide polychète sédentaire Nicolea zostericola. C. r. hedb. Séanc. Acad. Sci., Paris. 180: 864–866
Howie, D. I. D. (1961). The spawning of Arenicola marina. J. mar. biol. Assoc. U. K. 41: 127–144
Hsieh, H.-L., Simon, J. L. (1990). The sperm transfer system in Kinbergonuphis simoni (Polychaeta: Onuphidae). Biol. Bull. mar. biol. Lab., Woods Hole 178: 85–93
Jamieson, B. G. M., Afzelius, B. A., Franzén, Å. (1985). Ultrastructure of the acentriolar, aflagellate spermatozoa and the eggs of Histriobdella homari and Stratiodrilus novaehollandiae (Histriobdellidae, Polychaeta). J. submicrosc. Cytol. 17: 363–380
Jamieson, B. G. M., Rouse G. W. (1989). The spermatozoa of the Polychaeta (Annelida): an ultrastructural review. Biol. Rev. 64: 93–157
Jamieson, B. G. M., Webb, R. I. (1984). The morphology, spermatozoal ultrastructure and phylogenetic affinities of a new species of questid (Polychaeta: Annelida). In: Hutchings, P. (ed.) Proceedings of the first international polychaete conference. Linnean Society of N. S. W., Sydney, p. 21–34
Mesnil, F. (1897). Études de morphologie externe chez les Annélides. III. Formes intermédiares les Arénicoliens. Bull. scient. Fr. Belg. 30: 144–165
Newell, G. E. (1948). A contribution to the knowledge of the life history of Arenicola marina. J. mar. biol. Assoc. U. K. 27: 554–580
Newell, G. E. (1951). The life history of Clymenella torquata (Leidy). (Polychaeta). Proc. zool. Soc. Lond. 121: 561–586
Ô Foighil D. (1989). Role of spermatozeugmata in the spawning ecology of the brooding oyster Ostrea edulis. Gamete Res. 24: 219–228
Okada, K. (1941). The gametogenesis, the breeding habits, and the early development of Arenicola cristata Stimpson, a tubicolous polychaete. Sci. Rep. Tôhoku Univ. (Ser. 4) 16: 99–145
Okuda, S. (1938). Notes on the spawning habit of Arenicola claparedii Levinson. Annotnes zool. jap. 17: 577–580
Okuda, S. (1946). Studies on the development of Annelida Polychaeta I. J. Fac. Sci. Hokkaido Univ. (Ser. 6) 9: 115–219
Olive, P. J. W. (1985). Covariability of reproductive traits in marine invertebrates: implications for the phylogeny of the lower invertebrates. In: Conway Morris, S., George D., Gibson, R., Platt, H. M. (eds.) The origins and relationships of lower invertebrates. Oxford University Press, Oxford, p. 42–59
Pilgrim, M. (1964). The functional anatomy of the reproductive systems of the polychaetes Clymenella torquata and Caesicirrus neglectus. Proc. zool. Soc. Lond. 143: 443–464
Retzius, G. (1904). Zur Kenntnis der Spermien der Envertebraten. I. Biol. Unters. von G. Retzius, Neue Folge 11: 1–32
Reynolds, A. R. (1963). The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J. Cell Biol. 17: 208–212
Rice, S. A. (1978). Spermatophores and sperm transfer in spionid polychaetes. Trans. Am. microsc. Soc. 97: 160–170
Rice, S. A. (1981). Spermatophores and sperm ultrastructure in three species of Polydora and in Streblospio benedicti (Polychaeta: Spionidae). Zoomorphology 97: 1–16
Rouse, G. W. (1990). Four new species of Micromaldane (Maldanidae: Polychaeta) from eastern Australia. Rec. Aus. Mus. 42: 209–219
Rouse, G. W. (1992). Ultrastructure of spermatozoa of Oriopsis spp. (Sabellidae; Polychaeta). Zool. Scr. (in press)
Rouse, G. W., Jamieson, B. G. M. (1987). An ultrastructural study of the spermatozoa of the polychaetes Eurythoe complanata (Amphinomidae), Clymenella sp. and Micromaldane sp. (Maldanidae), with definitions of sperm types in relation to reproductive biology. J. submicrosc. Cytol. 19: 573–584
Schroeder, P. C., Hermans, C. O. (1975). Annelida: Polychaeta. In: Giese, A. C., Pearse, J. S. (eds.) Reproduction of marine invertebrates. III. Annelids and echiurans. Academic Press, New York, p. 1–213
Simon, J. L. (1967). Reproduction and larval development of Spio setosa (Spionidae; Polychaeta). Bull. mar. Sci. 17: 398–431
Werner, B. (1973). Spermatozeumen und Paarungsverhalten bei Tripedalia cystophora (Cubomedusae). Mar. Biol. 18: 212–217
Westheide, W. (1984). The concept of reproduction in polychaetes with small body size: adaptations in interstitial species. Fortschr. Zool. 29: 265–287
Westheide, W. (1988). Genital organs. In: Westheide, W., Hermans, C. O. (eds.) The ultrastructure of Polychaeta. Gustav Fischer Verlag, Stuttgart, p. 263–279
Wolf, P. S. (1983). A revision of the Boguidae Hartman and Fauchald, 1971, and its reduction to Bogueinae, a subfamily of Maldanidae (Polychaeta). Proc. biol. Soc. Wash. 96: 238–249
Author information
Authors and Affiliations
Additional information
Communicated by J. Grassle, New Brunswick
Rights and permissions
About this article
Cite this article
Rouse, G.W. Ultrastructure of sperm and spermathecae in Micromaldane spp. (Polychaeta: Capitellida: Maldanidae). Marine Biology 113, 655–668 (1992). https://doi.org/10.1007/BF00349709
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00349709