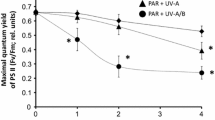
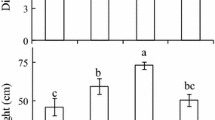
Abstract
Effects of high irradiance on photosynthetic characteristics were examined in sporophytes of the kelp Laminaria saccharina Lamour. from 1992 to 1994. Exposure to high irradiance (700 μmol photons m-2s-1) for 1 h at optimal temperature (12°C) caused a 40 to 60% decline in photosynthetic efficiency (alpha), quantum yield, and the ratio of variable to maximum chlorophyll fluorescence (Fv/Fm), an indicator of Photosystem II efficiency. Although the photoinhibition effects were partly attributable to protective mechanisms, a concurrent increase in minimal fluorescence (Fo) indicated damage to Photosystem II reaction centers. The magnitude of photoinhibition was proportional to irradiance and duration; however, Fv/Fm was significantly reduced after exposure to irradiances as low as 40 to 50 μmol photons m-2s-1 for 1 h, or to 700 μmol photons m-2s-1 for only 5 min. In contrast, photosynthetic capacity (Pmax) was affected only at much higher irradiance. Superoptimal temperatures up to 24°C did not exacerbate high-light effects. At 25°C, however, alpha and Pmax were more susceptible to photoinhibition than at lower temperatures. Recovery from photoinhibition was examined by following Fv/Fm and Fo for 24 h after exposure to high light. Recovery of Fv/Fm was fastest during the first 1 to 3 h, and slowed or ceased after 6 to 12 h, while recovery of Fo was relatively constant over 12 h. Dithiothreitol, which blocks formation of energy-dissipating xanthophylls, reduced both the initial rate and extent of recovery. Chloramphenicol, which blocks chloroplast-encoded protein synthesis, had little effect on initial rates of recovery, but stopped recovery after 3 h. Thus, L. saccharina appears to rely on the xanthophyll cycle to protect the photosynthetic apparatus, and reversal of this protective mechanism causes the rapid initial recovery in Fv/Fm. Longterm recovery depends on repair of damaged reaction centers. Both the rate and extent of recovery were temperature-dependent. The initial rate was higher at 18 to 22°C than at 12°C, but the extent of recovery over 24 h declined with increasing temperature. High temperatures, therefore, appear to enhance protective mechanisms, but disrupt repair processes. L. saccharina from Long Island Sound, an ecotype adapted to low light and high temperature, showed slightly but consistently greater effects of photoinhibition than plants from the Atlantic coast of Maine, but exhibited faster recovery at superoptimal temperatures.
Similar content being viewed by others
References
Anderson JM, Osmond CB (1987) Shade-sun responses: compromises between acclimation and photoinhibition. In: Kyle DJ, Osmond CB, Arntzen CJ (eds) Topics in photosynthesis. Vol. 9. Elsevier, Amsterdam, pp 1–38
Aro E-M, Virgin I, Andersson B (1993) Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim biophys Acta 1043: 113–134
Benet H, Bruss U, Duval J-C, Kloareg B (1994) Photosynthesis and photoinhibition in protoplasts of the marine brown alga Laminaria saccharina. J exp Bot 45: 211–220
Bilger W, Björkman O (1991) Temperature dependence of violaxanthin de-epoxidation and non-photochemical fluorescence quenching in intact leaves of Gossypium hirsutum L. and Malva parviflora L. Planta 184: 226–234
Bilger W, Björkman O, Thayer SS (1989) Light-induced spectral absorbance changes in relation to photosynthesis and the epoxidation state of xanthophyll cycle components in cotton leaves. Pl Physiol 91: 542–551
Björkman O, Demmig B (1987) Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77K among vascular plants of diverse origin. Planta 170: 489–504
Bolton JJ, Lüning K (1982) Optimal growth and maximal survival temperature of Atlantic Laminaria species (Phaeophyta) in culture. Mar Biol 66: 89–94
Bruhn J (1994) Photoinhibition in the common kelp, Laminaria saccharina. MS Thesis, State University New York, Stony Brook
Davison I (1991) Environmental effects on algal photosynthesis: temperature. J Phycol 27: 2–8
Demmig B, Björkman O (1987) Comparison of the effect of excessive light on chlorophyll fluorescence (77K) and photon yield of O2 evolution in leaves of higher plants. Planta 171: 171–184
Demmig-Adams B (1990) Carotenoids and photoprotection in plants: a role for xanthophyll zeaxanthin. Biochim biophys Acta 1020: 1–24
Dring MJ, Luning K (1994) Influence of spring-neap tidal cycles on the light available for photosynthesis by benthic marine plants. Mar Ecol Prog Ser 104: 131–137
Duval JC, Harker M, Rousseau B, Young AJ, Britton G, Lemoine Y (1992) Photoinhibition and zeaxanthin formation in the brown algae Laminaria saccharina and Pelvetia canaliculata. In: Murata N (ed) Research in photosynthesis. Vol. IV. Kluwer Academic Publications, Amsterdam, pp 581–584
Franklin L, Levavasseur G, Osmond CB, Henley WJ, Ramus J (1992) Two components of onset and recovery during photoinhibition of Ulva rotundata. Planta 186: 399–408
Gerard VA (1988) Ecotypic differentiation in light-related traits of the kelp Laminaria saccharina. Mar Biol 97: 25–36
Gerard VA (1990) Ecotypic differentiation in the kelp Laminaria saccharina: phase-specific adaptation in a complex life cycle. Mar Biol 107: 519–528
Gerard VA, Du Bois KR (1988) Temperature ecotypes near the southern boundary of the kelp Laminaria saccharina. Mar Biol 97: 575–580
Gerard VA, Du Bois KR, Greene R (1987) Growth responses of two Laminaria saccharina populations to environmental variation. Hydrobiologia 151/152: 229–232
Greene RM, Gerard VA (1990) Effects of high-frequency light fluctuations on growth and photoacclimation of the red alga Chondrus crispus. Mar Biol 105: 337–344
Greer DH, Berry JA, Björkman O (1986) Photoinhibition of photosynthesis in intact bean leaves: role of temperature, and requirement for chloroplast-protein synthesis during recovery. Planta 168: 253–260
Greer DH, Laing WA (1988) Photoinhibition of photosynthesis in intact Kiwi fruit, Actinidia deliciosa, leaves: effect of temperature. Planta 174: 159–165
Greer DH, Ottander C, Öquist G (1991) Photoinhibition and recovery of photosynthesis in intact barley leaves at 5 and 20°C. Physiologia Pl 81: 229–239
Hanelt D (1992) Photoinhibition of photosynthesis in marine macrophytes of the South China Sea. Mar Ecol Prog Ser 82: 199–206
Hanelt D, Huppertz K, Nultsch W (1992) Photoinhibition of photosynthesis and its recovery in red algae. Botanica Acta (Ber dt bot Ges) 105: 278–279
Hanelt D, Li J, Nultsch W (1994) Tidal dependence of photoinhibition of photosynthesis in marine macrophytes of the South China Sea. Botanica Acta (Ber dt bot ges) 107: 66–72
Hanelt D, Uhrmacher S, Nultsch W (1995) The effect of photoinhibition on photosynthetic oxygen production in the brown alga Dictyota dichotoma. Botanica Acta (Ber dt bot Ges) 108: 99–105
Henley WJ (1993) Measurement and interpretation of photosynthetic light-response curves in algae in the context of photoinhibition and diel changes. J Phycol 29: 729–739
Henley WJ, Lindley ST, Levavasseur G, Osmond CB, Ramus J (1992) Photosynthetic response of Ulva rotundata to light and temperature during emersion on an intertidal sand flat. Oecologia 89: 516–523
Henley WJ, Ramus J (1989) Photoacclimation and growth rate responses of Ulva rotundata (Chlorophyta) to intraday variations in growth irradiance. J Phycol 25: 398–401
Herbert SK (1990) Photoinhibition resistance in the red alga Porphyra perforata. Pl Physiol 92: 514–519
Herbert SK, Waaland JR (1988) Photoinhibition of photosynthesis in a sun and shade species of the red algal genus Porphyra. Mar Biol 97: 1–7
Horton P, Noctor G, Rees D (1990) Regulation of light harvesting and electron transport in photosystem II. In: Zelitch I (ed) Perspectives in biochemical and genetic regulation of photosynthesis. Wiley-Liss, New York, pp 145–158
Huppertz K, Hanelt D, Nultsch W (1990) Photoinhibition of photosynthesis in the marine brown alga Fucus serratus as studied in field experiments. Mar Ecol Prog Ser 66: 175–182
Jensen S, Knutsen G (1993) Influence of light and temperature on photoinhibition of photosynthesis in Spirulina platensis. J appl Phycol 5: 495–504
Kain JM (1979) A review of the genus Laminaria. Oceanogr Mar Biol A Rev 17: 101–161
Kassim A-K, Paulsen G (1989) Enhancement of thermal injury to photosythesis in wheat plants and thylakoids by high light intensity. Pl Physiol 90: 1041–1048
Kyle DJ (1987) The biochemical basis for photoinhibition of photosystem II. In: Kyle DJ, Osmond CB, Arntzen CJ (eds) Topics in photosynthesis. Vol. 9. Elsevier, Amsterdam, pp 197–226
Leverenz JW, Falk S, Pilström C-M, Samuelsson G (1990) The effects of photoinhibition on the photosynthetic light response curve of green plant cells (Chlamydomonas reinhardtii). Planta 182: 161–168
Ludlow MM (1987) Light stress at high temperature. In: Kyle DJ, Osmond CB, Arntzen CJ (eds) Topics in photosynthesis. Vol. 9. Elsevier, Amsterdam, pp 89–109
Ludlow MM, Powles SB (1988) Effects of photoinhibition induced by water stress on growth and yield of grain sorghum. Aust J Pl Physiol 15: 179–194
Lüning K (1990) Seaweeds, their environment, biogeography, and ecophysiology. John Wiley and Sons, New York
Marra J (1978) Effect of short-term variations in light intensity on photosynthesis of a marine phytoplankter: a laboratory simulation study. Mar Biol 46: 191–202
McLachlan J (1979) Growth media — marine. In: Stein JR (ed) Handbook of phycological methods. Cambridge University Press, Cambridge, pp 25–51
Neale PJ, Melis A (1990) Activation of a reserve pool of photosystem II in Chlamydomonas reinhardtii counteracts photoinhibition. Pl Physiol 92: 1196–1204
Nultsch W, Pfau J, Materna-Weide M (1987) Fluence and wavelength dependence of photoinhibition in the brown alga Dictyota dichotoma. Mar Ecol Prog Ser 41: 93–97
Ögren E, Rosenqvist E (1992) On the significance of photoinhibition in the field and its generality among species. Photosynthesis Res 33: 63–71
Öquist G, Malmberg G (1989) Light and temperature dependent inhibition of photosynthesis in frost-hardened and unhardened seedlings of pine. Photosynthesis Res 20: 261–277
Osmond CB, Ramus J, Levavasseur G, Franklin LA, Henley WJ (1993) Fluorescence quenching during photosynthesis and photoinhibition of Ulva rotundata Blid. Planta 190: 97–106
Powles SB (1984) Photoinhibition of photosynthesis induced by visible light. A Rev Pl Physiol 35: 15–44
Prasil O, Adir N, Ohad I (1992) Dynamics of photosystem II: mechanism of photoinhibition and recovery processes. In: Barber J (ed) Topics in photosynthesis. Vol. 11. Elsevier, Amsterdam, pp 293–348
Ramus J, Rosenberg G (1980) Diurnal photosynthetic performance of seaweeds measured under natural conditions. Mar Biol 56: 21–28
Raven JA, Richardson K (1986) Marine environments. In: Baker N, Long S (eds) Photosynthesis in contrasting environments. Elsevier, Amsterdam, pp 337–398
Samuelsson G, Lönnberg A, Gustafsson P, Öquist G (1987) The susceptibility of photosynthesis to photoinhibition and the capacity of recovery in high and low light grown cyanobacterium, Anacystis nidulans. Pl Physiol 83: 438–441
Uhrmacher S, Hanelt D, Nultsch W (1995) Zeaxanthin content and the degree of photoinhibition are linearly correlated in the brown alga Dictyota dichotoma. Mar Biol 123: 159–165
Vonshak A, Torzillo G, Tomaseli L (1994) Use of chlorophyll fluorescence to estimate the effect of photoinhibition in outdoor cultures of Spirulina platensis. J appl Phycol 6: 31–34
Author information
Authors and Affiliations
Additional information
Communicated by J. P. Grassle, New Brunswick
Rights and permissions
About this article
Cite this article
Bruhn, J., Gerard, V.A. Photoinhibition and recovery of the kelp Laminaria saccharina at optimal and superoptimal temperatures. Marine Biology 125, 639–648 (1996). https://doi.org/10.1007/BF00349245
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00349245