Abstract
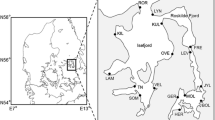
Limited dispersal should result in genetic differences between populations proportional to geographic distances of separation. This association between gene flow and distance can be disrupted by (1) continuing genetic exchange among distant populations, (2) historical changes in gene flow, and (3) physical barriers or corridors to dispersal. The movements of larvae are thought to determine dispersal capability in benthic marine invertebrates. The solitary scleractinian Balanophyllia elegans Verrill possesses crawling larvae capable of only limited dispersal. Paradoxically, however, inferred levels of gene flow between pairs of localities spread over much of the 4000 km range of B. elegans exhibited a weaker relationship with geographical separation than that expected for a linear array of populations in which all genetic exchange takes place between adjacent populations. In this paper, I examined the pattern of gene flow (inferred from the frequencies of eight polymorphic allozyme loci) in B. elegans at a smaller (1 to 50 km) spatial scale to determine (1) whether gene flow at this spatial scale conformed to the expectations of the stepping-stone model, and (2) whether continuing long-distance gene flow or historical changes in gene flow were responsible for the weak relationship between gene flow and distance observed previously at the rangewide spatial scale. Between May and August 1992, I collected 75 adults from each of 18 localities along the coast of Sonoma County, California, USA. These populations of B. elegans were significantly subdivided both among localities separated by 1 to 50 km (F LT =0.053, Se=0.0075) and among patches separated by 4 to 8 m (F PL=0.026, SE=0.0023). The observed slope and correlation (r 2=0.54) between inferred levels of gene flow and the geographic distance at the 1 to 50 km spatial scale conformed to equilibrium expectations (obtained by simulation) for a linear stepping-stone model, although those from the rangewide spatial scale did not. This implies that the mechanisms conferring patterns of inferred genetic differentiation between localities in B. elegans differ fundamentally with spatial scale. At a scale of 1 to 50 km, continuing gene flow and drift have equilibrated and the process of isolation-bydistance may facilitate local adaptive change. At a broader spatial scale, historical changes in gene flow, perhaps affected by late Pleistocene climatic fluctuations, disrupt the equilibration of gene flow and genetic drift, so that genetic differentiation may not increase continuously with separation between populations.
Similar content being viewed by others
References
Archie JW (1985) Statistical analysis of heterozygosity data: independent sample comparisons. Evolution 39:623–637
Ayre DJ (1991) Population subdivision in Australian temperate marine invertebrates: larval connections versus historical factors. Aust J Ecol 15:403–411
Behrens Yamada S (1989) Are direct developers more locally adapted than planktonic developers? Mar Biol 103:403–411
Bell G (1992) Five properties of the environment. In: Grant PR, Horn HS (eds) Molds, molecules, and evolution. Princeton, pp 33–56
Burton RS (1986) Evolutionary consequences of restricted gene flow among natural populations of the copepod Tigriopus californicus. Bull mar Sci 39:526–535
Cockerham CC, Weir BS (1993) Estimation of gene flow from F-statistics. Evolution 47:855–863
Crow JF, Aoki K (1984) Group selection for a polygenic behavioral trait: estimating the degree of population subdivision. Proc natn Acad Sci USA 81:6073–6077
Durham JW, Barnard JL (1952) Stony corals of the Eastern Pacific collected by the Velero III and Velero IV. Allan Hancock Pacif Exped 16:1–110
Endler JA (1973) Gene flow and population differentiation. Science, NY 179:243–250
Fadlallah YH (1983) Population dynamics and life history of a solitary coral, Balanophyllia elegans, from Central California. Oecologia 58:200–207
Fadlallah YH, Pearse JS (1982) Sexual reproduction in solitary corals: overlapping oogenic and brooding cycles, and benthic planulas in Balanophyllia elegans. Mar Biol 71:223–231
Gerrodette T (1979a) Ecological studies of two temperate solitary corals. Ph.D. thesis, Scripps Institution of Oceanography, University of Calif, San Diego
Gerrodette T (1979b) Equatorial submergence in a solitary coral, Balanophyllia elegans, and the critical life stage excluding the species from shallow water in the south. Mar Ecol Prog Ser 1:227–235
Gerrodette T (1981) Dispersal of the solitary coral Balanophyllia elegans by demersal planular larvae. Ecology 62:611–619
Hansen TA (1978) Larval dispersal and species longevity in Lower Tertiary gastropods. Science,NY 199:885–887
Hellberg ME (1994) Relationships between inferred levels of gene flow and geographic distance in a philopatric coral, Balanophyllia elegans. Evolution 48:1829–1854
Jablonski D (1986) Larval ecology and macroevolution in marine invertebrates. Bull mar Sci 39:565–587
Janson K (1983) Selection and migration in two distinct phenotypes of Littorina saxatilis in Sweden. Oecologia 59:58–61
Janson K, Ward RD (1984) Microgeographical variation in allozyme and shell characters in Littorina saxatilis Olivi (Prosobranchia: Littorinidae). Biol J Linn Soc 22:289–307
Johannesson K, Johannesson B (1989) Differences in allele frequencies of Aat between high- and mid-rocky shore populations of Littorina saxatilis (Olivi) suggest selection in this enzyme locus. Genet Res 54:7–11
Johnson MS, Black R (1991) Genetic subdivision of the intertidal snail Bembicium vittatum (Gastropoda: Littorinidae) varies with habitat in the Houtman Abrolhus Islands, Western Australia. Heredity, Lond 67:205–213
Kimura M, Weiss GH (1964) The stepping stone model of population structure and the decrease of genetic correlation with distance. Genetics, Austin, Tex 49:561–576
Knowlton N, Jackson JBC (1993) Inbreeding and outbreeding in marine invertebrates. In: NW Thornhill (ed) The natural history of inbreeding and outbreeding: theoretical and empirical perspectives. University of Chicago, Chicago, Illinois, pp 200–249
Koehn RK, Newell RIE, Immermann F (1980) Maintenance of an aminopeptidase allele frequency cline by natural selection. Proc natn Acad Sci USA 77:5385–5389
Larson A, Wake DB, Yanev KP (1984) Measuring gene flow among populations having high levels of genetic fragmentation. Genetics, Austin, Tex 106:293–308
Leberg PL (1992) Effects of population bottlenecks on genetic diversity as measured by allozyme electrophoresis. Evolution 46:477–494
Lindberg DR (1991) Marine biotic interchange between northern and southern hemisphere. Paleobiology 17:308–324
McArdle BH (1988) The structural relationship: regression in biology. Can J Zool 66:2329–2339
Nei M (1973) Analysis of gene diversity in subdivided populations. Proc natn Acad Sci USA 70:3321–3323
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics, Austin, Tex 89:583–590
Reid DG (1990) Trans-arctic migration and speciation induced by climatic change: the biogeography of Littorina (Mollusca: Gastropoda). Bull mar Sci 47:35–49
Scheltema RS (1977) Dispersal of marine invertebrate organisms: paleobiogeographic and biostrategraphic implications. In: Kaufman EG, and Hazel JE (eds) Concepts and methods of biostratigraphy. Dowden, Hutchinson & Ross, Stroudsburg, Pennsylvania, pp 73–108
Selander RK, Smith MH, Yang SY, Johnson WE, Gentry JB (1971) Biochemical polymorphism and systematics in the genus Peromyscus. I. Variation in the old-field mouse (Peromyscus polionotus). Stud Genet, Austin, Tex 6:49–90
Shuto T (1974) Larval ecology of prosobranch gastropods and its bearing on biogeography and paleontology. Lethaia 7:239–256
Slatkin M (1985) Gene flow in natural populations. A Rev Ecol Syst 16:393–430
Slatkin M (1991) Inbreeding coefficients and coalescence times. Genet Res 58: 167–175
Slatkin M (1993) Isolation by distance in equilibrium and nonequilibrium populations. Evolution 47:264–279
Slatkin M, Barton NH (1989) A comparison of three indirect methods for estimating average levels of gene flow. Evolution 43:1349–1368
Slatkin M, Maddison WP (1990) Detecting isolation by distance using phylogenies of genes. Genetics, Austin, Tex 126:249–260
Swofford DL, Selander RK (1989) BIOSYS-1: a computer program for the analysis of allelic variation in population genetics and biochemical systematics. University of Illinois, Urbana, Illinois
Valentine JW, Jablonski D (1983) Speciation in the shallow sea: general patterns and biogeographic controls. In: Sims RW, Price JH, Whalley PES (eds) Evolution, time and space: the emergence of the biosphere. Academic Press, New York, pp 201–226
Vermeij GJ (1989) Geographical restriction as a guide to the causes of extinction: the case of the cold northern oceans during the Neogene. Paleobiology 15:335–356
Ward RD, Beardmore JA (1977) Protein variation in the plaice (Pleuronectes platessa). Genet Res 30:45–62
Weir BS (1990) Intraspecific differentiation. In: Hillis DM, Moritz C (eds) Molecular systematics. Sinauer, Sunderland, Massachusetts, pp 373–410
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Wright S (1943) Isolation by distance. Genetics, Princeton, 28:114–138
Wright S (1951) The genetical structure of populations. Ann Eugen 15:323–354
Wright S (1978) Evolution and the genetics of populations. Vol 4. Variability within and among natural populations. University of Chicago, Press Chicago
Author information
Authors and Affiliations
Additional information
Communicated by M.F. Strathmann, Friday Harbor
Rights and permissions
About this article
Cite this article
Hellberg, M.E. Stepping-stone gene flow in the solitary coral Balanophyllia elegans: equilibrium and nonequilibrium at different spatial scales. Marine Biology 123, 573–581 (1995). https://doi.org/10.1007/BF00349236
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00349236