Abstract
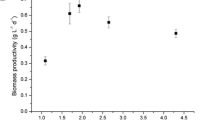
Rhodobacter capsulatus strain 37b4 was grown phototrophically in chemostat cultures with 2 mM of ammonium chloride and 30 mM of malate at a constant dilution rate of 0.075 h-1. When illumination was raised from 3000 to 30000 lx, steady state biomass levels as well as malate uptake increased linearly with increasing illumination. Yet, in no case external ammonium could be detected in the culture fluid. Specific nitrogenase activity increased by a factor of ten between 3000 and 15000 lx and approached constancy above 15 000 lx. When samples were anaerobically withdrawn from the chemostat and subsequently grown in batch cultures under saturating light conditions, biomass increased to a constant level, independently of the illumination used in the previous chemostat culture. In fact, the specific nitrogen contents of cells were 0.195 and 0.154 (g of N per g of protein) with chemostat cultures adapted to 3000 and 30000 lx, respectively. With the former cultures, specific nitrogen contents decreased to 0.142 g of nitrogen per g of cell protein upon incubation in a batch system. This suggests the existence of free nitrogen compounds in cells of chemostat cultures, the concentrations of which decrease while protein levels increase with increasing energy supply. Intracellular amino acid pools revealed slightly elevated levels of major amino acids in low-light cultures as compared to high-light cultures. On the basis of intracellular levels of ammonium, however, no significant differences could be detected. Since, in addition, malate consumption increased linearly with increasing illumination, it is proposed that light controls nitrogenase in Rhodobacter capsulatus via the C/N ratio, as represented by malate and ammonium consumption, rather than directly.
Similar content being viewed by others
References
Beloserski AN, Proskurjakow L (1956) Praktikum der Biochemie der Pflanzen. Deutscher Verlag der Wissenschaften, Berlin
Dingler C, Kuhla J, Wassink H, Oelze J (1988) Levels and activities of nitrogenase proteins in Azotobacter vinelandii grown at different dissolved oxygen concentrations. J Bacteriol 170: 2148–2152
Grether-Beck S, Oelze J (1987) The development of the photosynthetic apparatus and energy transduction in malatelimited phototrophic cultures of Rhodobacter capsulatus. Arch Microbiol 149: 70–75
Hallenbeck PC (1987) Molecular aspects of nitrogen fixation by photosynthetic prokaryotes. In: O'Leary WM (ed) CRC Crit Rev Microbil 14: 1–48
Hallenbeck PC, Meyer CM, Vignais PM (1982) Nitrogenase from the photosynthetic bacterium Rhodopseudomonas capsulata: purification and molecular properties. J Bacteriol 149: 708–717
Hawkes R, Niday E, Gordon J (1982) A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem 119: 142–147
Herbert RA, Siefert E, Pfennig N (1980) Utilization of l-alanine by Rhodopseudomonas acidophila. Arch Microbiol 125: 105–109
Hoover TR, Ludden PW (1984) Derepression of nitrogenase by addition of malate to cultures of Rhodospirillum rubrum grown with glutamate as the carbon and nitrogen source. J Bacteriol 159: 400–403
Jouanneau Y, Lebeque S, Vignais PM (1984) Ammonia and light effect on nitrogenase activity in nitrogen-limited continuous cultures of Rhodopseudomonas capsulata. Role of glutamine synthetase. Arch Microbiol 139: 326–331
Jouanneau Y, Wong B, Vignais PM (1985) Stimulation by light of nitrogenase synthesis in cells of Rhodopseudomonas capsulata growing in N-limited continuous cultures. Biochim Biophys Acta 808: 149–155
Kanemoto RH, Ludden PW (1984) Effect of ammonia, darkness, and phenazine methosulfate on whole-cell nitrogenase activity ER, Chock PB, Levitzki A (eds) Current topics in cellular regulation, vol 30. Academic Press, San Diego, pp 23–56
Moreno-Vivián C, Caballero FJ, Cárdenas J, Castillo F (1989) Effect of the C/N balance on the regulation of nitrogen fixation in Rhodobacter capsulatus E1F1. Biochim Biophys Acta 977: 297–300
Pollock D, Bauer CE, Scolnik PA (1988) Transcription of the Rhodobacter capsulatus nifHDK operon is modulated by the nitrogen source. Construction of plasmid expression vectors based on the nifHDK promoter. Gene 65: 269–275
Postgate JR (1982) The fundamentals of nitrogen fixation. University Press. Cambridge
Steinborn B, Oelze J (1989) Nitrogenase and photosynthetic activities of chemostat cultures of Rhodobacter capsulatus 37b4 grown under different illuminations. Arch Microbiol 152: 100–104
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedures and some applications. Proc Natl Acad Sci USA 76: 4350–4354
Weaver PF, Wall JD, Gest H (1975) Characterization of Rhodopseudomonas capsulata. Arch Microbiol 105: 207–216 and Fe protein modification in Rhodospirillum rubrum. J Bacteriol 158: 713–720
Kanemoto RH, Ludden PW (1987) Amino acid concentrations in Rhodospirillum rubrum during expression and switch-off of nitrogenase activity. J Bacteriol 169: 3035–3043
Kleiner D (1976) Ammonium uptake and metabolism by nitrogenfixing bacteria. II. Klebsiella pneumoniae. Arch Microbiol 111: 85–91
Kranz RG, Foster-Hartnett D (1990) Transcriptional regulatory cascade of nitrogen-fixation genes in anoxygenic photosynthetic bacteria: oxygen- and nitrogen-responsive factors. Molecular Microbiology 4: 1793–1800
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685
Li J, Hu C-Z, Yoch DC (1987) Changes in amino acid and nucleotide pools of Rhodospirillum rubrum during switch-off of nitrogenase activity initiated by NH sup+inf4 or darkness. J Bacteriol 169: 231–237
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275
Ludden PW, Roberts GP (1989) Regulation of nitrogenase activity by reversible ADP ribosylation. In: Horecker BL, Stadtman
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Steinborn, B., Jürgens, U.J. & Oelze, J. Control of nitrogenase in chemostat cultures of Rhodobacter capsulatus grown on ammonium at different illuminations. Arch. Microbiol. 156, 135–141 (1991). https://doi.org/10.1007/BF00290986
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00290986