Summary
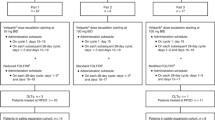
Based on the synergy of sequential methotrexate (MTX) and 5-fluorouracil (5-FU) in vitro and in vivo and the superior antitumor activity of trimetrexate (TMTX) compared with MTX in preclinical models, we carried out a phase I trial of TMTX and 5-FU (fixed dose, 400 mg/m2 per day), both given as 10-min i.v. infusions daily x5 days, every 28 days. The TMTX dose was escalated from 3.0 to 14.0 mg/m2 per day. In all, 92 evaluable courses were given to 34 patients, half of whom were heavily pretreated with radiation or cytotoxics. Myelosuppression and mucositis were the dose-limiting toxicities but were not different in heavily or minimally pretreated patients; there were five episodes of moderate to severe mucositis. Rash, fatigue, and diarrhea were mild toxicities. Plasma TMTX elimination was biexponential, with a mean t.1/2α of 0.23 h and a t.1/2β of 16.7 h. The area under the plasma TMTX concentration versus time curve increased linearly with dose, suggesting first-order elimination. Total plasma TMTX clearance (mean±SD) was 14.3±5.9 ml/min per m2. Renal clearance accounted for approximately 7% of total clearance, indicating biotransformation as the major route of elimination. TMTX was highly protein-bound (97%). Thus, TMTX can be given with 5-FU (400 mg/m2) on a daily x 5-day bolus schedule at the 12 mg/m2 per day dose level, which was the recommended dose of TMTX as a single agent for phase II studies using the 5-day bolus schedule.
Similar content being viewed by others
References
Ackerly CA, Hartshorn J, Tong WP, McCormack JJ (1985) A rapid and sensitive method for determination of trimetrexate from biological fluids. J Liq Chromatogr 8: 125–134
Balis FM, Lester CM, Poplack DG (1986) Pharmacokinetics of trimetrexate (NSC 352122) in monkeys. Cancer Res 46: 169–174
Balis FM, Patel R, Luks E, Doherty K, Holcenberg JS, Tan C, Reaman GH, Belasco J, Ettinger LJ, Zimm S, Polpack DG (1987) Pediatric phase I trial and pharmacokinetic study of trimetrexate. Cancer Res 47: 4973–4976
Benz C, Cadman E (1981) Modulation of 5-fluorouracil metabolism and cytotoxicity by antimetabolite pretreatment in human colorectal adenocarcinoma HCT-8. Cancer Res 41: 994–999
Brown R, Manno J (1978) ESTRIP, a BASIC computer program for obtaining initial polyexponential parameter estimates. J Pharm Sci 67 (12): 1687–1691
Cadman E, Heimer R, Davis L (1979) Enhanced 5-fluorouracil nucleotide formation after methotrexate administration: explanation for drug synergism. Science 205: 1135–1137
Fanucchi M, Walsh TD, Fleisher M, Lokos G, Williams L, Cassidy C, Vidal P, Chou TC, Niedzwiecki D, Young CW (1987) Phase I and clinical pharmacology study of trimetrexate administered weekly for three weeks. Cancer Res 47: 3303–3308
Gibaldi M, Perrier D (1982) Pharmacokinetics, 2nd edn. Marcel Dekker, New York
Ho DH, Covington WP, Legha SS, Newman RA, Krakoff IH (1987) Clinical pharmacology of trimetrexate. Clin Pharmacol Ther 42(3): 351–356
Jackson RC, Fry DW, Boritzki TJ, Besserer JA, Leopdd WR, Sloan BJ, Elslager EF (1984) Biochemical pharmacology of the lipophilic antifolate, trimetrexate. Adv Enzyme Regul 22: 187–206
Kamen BA, Cashmore AR, Dreyer RN, Bertino JR (1984) Uptake and efficacy of trimetrexate, a non-classical antifolate, in methotrexate-resistant leukemia cells in vitro. Biochem Pharmacol 33: 1697
Latham B, Von Hoff DD, Elslager E (1984) Use of a human tumor cloning system to evaluate analogs of methotrexate and mitoxantrone. Cancer Treat Rep 68: 733–738
Leopold WR, Dykes DJ, Griswold DP (1987) Therapeutic synergy of trimetrexate (CI-898) in combination with doxorubicin, vincristine, cytoxan, 6-thioguanine, cisplatin, or 5-fluorouracil against intraperitoneally implanted P388 leukemia. Natl Cancer Inst Monogr 5: 99
Leyland-Jones B, O'Dwyer P (1986) Biochemical modulation: application of laboratory models to the clinic. Cancer Treat Rep 70: 219–229
Lin JT, Cashmore AR, Baker M, Dreyer RN, Ernstoff M, March JC, Bertino JR, Whitfield LR, Delap RJ, Grillo-Lopez A (1987) Phase I studies with trimetrexate: clinical pharmacology, analytical methodology, and pharmacokinetics. Cancer Res 47: 609–616
Mulder JH, Smink T, Van Putten LM (1981) 5-fluorouracil and methotrexate combination chemotherapy: the effect of drug scheduling. Eur J Cancer Clin Oncol 17: 831–837
National Cancer Institute (1983) Clinical brochure for trimetrexate glucuronate. NCI, Bethesda, Md
O'Dwyer PJ, Shoemaker DD, Plowman J, Cradock J, Grillo-Lopez A, Leyland-Jones B (1985) Trimetrexate: a new antifol entering clinical trials. Invest New Drugs 3: 71
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5: 649–655
Statistical Consultants, Inc. (1986) PCNONLIN and NONLIN84: software for the statistical analysis of nonlinear models. Am Stat 40 (1)
Stewart JA, McCormack JJ, Tong W, Low JB, Roberts JD, Blow A, Whitfield LR, Haugh LD, Grove WR, Grillo-Lopez AJ, Delap RJ (1988) A phase 1 clinical and pharmacokinetic study of trimetrexate using a daily x 5 schedule. Cancer Res 48: 5029–5035
Weiss RB, James WD, Major WB, Porter MB, Allegra CJ, Curt GA (1986) Skin reactions induced by trimetrexate, an analog of methotrexate. Invest New Drugs 4: 159–163
WHO (1979) Handbook for reporting results of cancer treatment. Offset Publication 48. WHO, Geneva
Yamaoka K, Nakagawa T, Uno T (1978) Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm 6 (2): 165–175
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hudes, G.R., LaCreta, F., DeLap, R.J. et al. Phase I clinical and pharmacologic trial of trimetrexate in combination with 5-fluorouracil. Cancer Chemother. Pharmacol. 24, 117–122 (1989). https://doi.org/10.1007/BF00263132
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00263132