Abstract
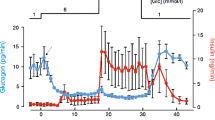
The G-protein-mediated coupling of a glucagon receptor to ATP-dependent K channels—KATP—has been studied in insulin-secreting cells using the patch clamp technique. In excised outside-out patches, KATP channel activity was inhibited by low concentrations of glucagon (IC50 = 2.4 nm); the inhibitory effect vanished at concentrations greater than 50 nm. In cell-attached patches, inhibition by bath-applied glucagon was seen most often, although stimulation was observed in a few cases. A dual action of the hormone is proposed to resolve these apparently divergent results. In excised inside-out patches, KATP channel activity was inhibited by addition of βγ subunits purified from either erythrocyte or retina (IC50 = 50 pm and 1 nm, respectively). Subsequent exposure of the patch to α i or α o reversed this effect. In excised inside-out patches, increasing Mg2+ in the bath stimulated the channel activity between 0 and 0.5 mm, but blocked it at higher concentrations (IC50 = 2.55 mm). In most cases (70%), GTP had a stimulatory effect at concentrations up to 100 μm. However, in three cases, similar GTP levels had clear inhibitory effects. In excised inside-out patches, cholera toxin (CTX) caused channel inhibition. Although the effect could not be reversed by removal of the toxin, the activity was restored by subsequent addition of purified α i or α o . These results are compatible with a model whereby channel inhibition by activated G S -coupled receptors occurs, at least in part, via association of the βγ subunits of G S with α i /α o subunits and deactivation of the α i /α o -dependent stimulatory pathway. On the basis of this hypothesis, a model is developed to describe the effects of G proteins on the KATP channel, as well as to account for the concentration-dependent stimulation and inhibition of KATP channel by Mg2+. An interpretation of the ability of glucagon to potentiate, but not initiate, insulin release is also given in terms of this model and the effects of ATP on KATP channels.
This work was supported by grant DCB-89 19368 from the National Science Foundation and a research grant (W-P 880513) from the American Diabetes Association to B.R.
Similar content being viewed by others
References
Ashcroft, F.M., Kakei, M. 1989. ATP-sensitive K+ channels in rat pancreatic β-cells: Modulation by ATP and Mg2+ ions. J. Physiology 416:349–367
Ashcroft, S.J.H., Weerasinghe, L.C.C., Randhall, P.J. 1973. Interrelationship of islet metabolism, adenosine triphosphate content and insulin release. Biochem. J. 132:223–231
Bezanilla, F. 1985. A high capacity data recording device based on a digital audio processor and a video cassette recorder. Biophys. J. 47:437–441
Birnbaumer, L., Abramowitz, J., Brown, A.M. 1990. Receptor-effector coupling by G proteins. Biochim. Biophys. Acta 1031:163–224
Bokoch, G.M., Katada, T., Northup, J.K., Ui, M., Gilman, A.G. 1984. Purification and properties of the inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. J. Biol. Chem. 259(6):3560–3567
Cerione, R.A., Codina, J., Kilpatrick, B.F., Staniszewski, C., Gierschik, P., Somers, R.L., Spiegel, A.M., Birnbaumer, L., Caron, M., Lefkowitz, R.J. 1985a. Transducin and the inhibitory nucleotide regulatory protein inhibit the stimulatory nucleotide regulatory protein mediated stimulation of adenylate cyclase in phospholipid vesicle systems. Biochemistry 24:4499–4503
Cerione, R.A., Staniszewski, C., Caron, M.G., Lefkowitz, R.J., Codina, J., Birnbaumer, L. 1985b. A role for Ni in the hormonal stimulation of adenylate cyclase. Nature 318:293–295
Codina, J., Hildebrandt, J.D., Sekura, R.D., Birnbaumer, M., Bryan, J., Manclark, C.R., Lyengar, R., Birnbaumer, L. 1984. Ns and Ni, the stimulatory and inhibitory regulatory components of adenylate cyclases. J. Biol. Chem. 259(9):5871–5886
Colquhoun, D., Sigworth, F.J. 1983. Fitting and statistical analysis of single channel records. In: Single-Channel Recording. B. Sakmann and E. Neher, editors, pp. 191–263. Plenum, New York-London
Dunne, M.J., Petersen, O.H. 1986. GTP and GDP activation of K+ channels that can be inhibited by ATP. Pfluegers Arch. 407:564–565
Fehmann, H.-C., Habener, J.F. 1992. Insulinotropic hormone glucagon-like peptide-I (7–37) stimulation of proinsulin gene expression and proinsulin biosynthesis in insulinoma βTC-1 cells. Endocrinology 130(1):159–166
Findlay, I. 1987. The effect of magnesium upon adenosine triphosphate-sensitive potassium channels in a rat insulin-secreting cell line. J. Physiol. 391:611–629
Goldfine, I.D., Roth, J., Birnbaumer, L. 1972. Glucagon receptor in β-cells. J. Biol. Chem. 247(4): 1211–1218
Hamill, O.P., Marty, A., Neher, E., Sakmann, B., Sigworth, F.J. 1981. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pfluegers Arch. 391:85–100
Holz, G.G., IV, Kuhtreiber, W.M., Habener, J.F. 1993. Pancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1 (7–37). Nature 361:362–365
Hoosein, N.M., Gurd, R.S. 1984. Human glucagon-like peptides 1 and 2 activate rate brain adenylate cyclase. FEBS Lett. 178(1):83–86
Ito, H., Tung, R.T., Sugimoto, T., Kobayashi, I., Takahashi, K., Katada, T., Ui, M., Kurachi, Y. 1992. On the mechanism of G protein βγ subunit activation of the muscarinic K+ channel in guinea pig atrial cell membrane. J. Gen. Physiol. 99:961–983
Katada, T., Bokoch, G.M., Northup, J.K., Ui, M., Gilman, A.G. 1984. The inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. J. Biol. Chem. 259(6):3568–3577
Kofod, H., Andreu, D., Thams, P., Merrifield, R.B., Hedeskov, C.J., Hansen, B., Lernmark, A. 1988a. Insulin release by glucagon and secretin: studies with secretin-glucagon hybrids. Am. J. Physiol. 254:E454-E458
Kofod, H., Unson, C.G., Merrifield, R.B. 1988b. Potentiation of glucose-induced insulin release in islets by desHis1[Glu9]glucagon amide. Int. J. Prot. Res. 32:436–440
Murphy, G.J., Houslay, M.D. 1988. Resensitization of hepatocyte glucagon-stimulated adenylate cyclase can be inhibited when cyclic AMP phosphodiesterase inhibitors are used to elevate intracellular cyclic AMP concentrations to supraphysiological values. Biochem. J. 249:543–547
Northup, J.K., Smigel, M.D., Gilman, A.G. 1982. The guanine nucleotide activating site of the regulatory component of adenylate cyclase. J. Biol. Chem. 257:11416–11423
Okabe, K., Yatani, A., Evans, T., Ho, Y-K., Codina, J., Birnbaumer, L., Brown, A.M. 1990. βγ dimers of G proteins inhibit atrial muscarinic K+ channels. J. Biol. Chem. 265(22): 12854–12858
Pipeleers, D.G., Schuit, F.C., In'T Veld, P.A., Maes, E., Hooghepeters, M., Van De Winkel, M., Gepts, W. 1985. Interplay of nutrients and hormones in the regulation of insulin release. Endocrinology 117(3):824–833
Ribalet, B., Ciani, S., Eddlestone, G.T. 1989a. ATP mediates both activation and inhibition of K(ATP) channel activity via cAMP dependent protein kinase in insulin secreting cell lines. J. Gen. Physiol. 94:693–717
Ribalet, B., Ciani, S., Eddlestone, G.T. 1989b. Modulation of ATP-sensitive K channels in RINm5F cells by phosphorylation and G proteins. Biophys. J. 55:587a
Ribalet, B., Ciani, S., Hales, T.G., Eddlestone, G.T. 1991. Role of G proteins in K(ATP) channel modulation by the neuropeptide somatostatin. Biomed. Res. 12(2):37–40
Ribalet, B., Eddlestone, G.T., Ciani, S. 1988. Metabolic regulation of the K(ATP) and a Maxi-K(V) channel in the insulin secreting RINm5F cell. J. Gen. Physiol. 92:219–237
Ullrich, S., Wollheim, C.B. 1988. GTP-dependent inhibition if insulin secretion by epinephrine in permeabilized RINm5F cells. J. Biol. Chem. 263:8615–8620
Yatani, A., Codina, J., Brown, A.M., Birnbaumer, L. 1987. Direct activation of mammalian atrial muscarinic potassium channels by GTP regulatory protein Gk. Science 235:207–211
Author information
Authors and Affiliations
Additional information
The authors would like to thank Dr. A.E. Boyd, III for supplying the RINm5F and HIT cells, Drs. J. Codina and L. Birnbaumer for supplying the G protein and β subunits from erythrocyte, Dr. R.A. Cerione for supplying the G protein β subunit from retina, and Mrs. Satoko Hagiwara for preparing and maintaining the cell cultures.
Rights and permissions
About this article
Cite this article
Ribalet, B., Ciani, S. Characterization of the G protein coupling of a glucagon receptor to the KATP channel in insulin-secreting cells. J. Membarin Biol. 142, 395–408 (1994). https://doi.org/10.1007/BF00233444
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00233444