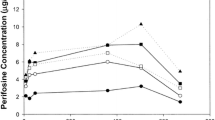
Summary
Thirty-three patients with advanced malignancy were treated with oral spirogermanium in a Phase I study to determine a maximum tolerated dose. Patients were entered in the study at doses of 100, 200, and 300 milligrams daily. The dose-limiting toxicity was gastrointestinal with moderate nausea and vomiting occurring with the 300 milligram dose. No myelosuppression or renal dysfunction was noted. Elevations of serum transaminase were seen in 41 percent of the patients at study entry. While abnormalities in hepatic function were recorded during the study, the relationship to spirogermanium could not be determined. No patient exhibited clinical hepatic dysfunction or elevation of serum bilirubin. It is recommended that future studies of oral spirogermanium incorporate careful monitoring of these parameters. Two patients with lymphoproliferative disorders had objective responses to therapy. A dose of 200 milligrams is recommended for further Phase II trials.
Similar content being viewed by others
References
Rice LM, Wheeler JW, Geschickter GF: Spirans XXII. Synthesis of 4,4-dialkyl-4-germacyclohexanone and 8,8-dialkyl8-germaazaspiro [4.5] decanes. J of Heterocyclic Chem 11:1041–1047, 1974
Hill BT, Whatley SA, Bellamy AS, Jenkins LY, Whelan RDH: Cytotoxic effects and biological activity of 2-aza-8-germanspiro [4.5] -decane-2-propanamine-8,8-diethyl-N,N-dimethyl dichloride (NSC 192965; Spirogermanium) in vitro. Cancer Res 42:2852–2856, 1982
Schein PS, Slavik M, Smythe T, Hoth D, Smith F, Macdonald JS, Woolley PV: Phase I clinical trial of spirogermanium. Cancer Treat Rep 64:1051–1056, 1980
Espana P, Kaplan R, Robichaud K, Gustafson P, Wiernik P, Smith F, Woolley P, Schein P: Phase II study of spirogermanium in lymphoma patients. (Abstr) Proc Am Soc Clin Oncol 1:166, 1982
Woolley PV, Ahlgren JP, Byrne PJ, Priego VM, Schein PS: A Phase I trial of spirogermanium administered on a continuous infusion schedule. Invest New Drugs 2:302–306, 1984
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Harvey, J., McFadden, M., Smith, F.P. et al. Phase I study of oral spirogermanium. Invest New Drugs 8, 53–56 (1990). https://doi.org/10.1007/BF00216924
Issue Date:
DOI: https://doi.org/10.1007/BF00216924