Abstract
Data on the absolute bioavailability of flecainide are controversial. We have investigated whether differences in metabolic clearances and/or the absorption profile might be responsible for the variability in its absolute bioavailability. Six subjects with a wide range of flecainide metabolic clearances (85–407 ml·min−1) simultaneously received the drug by the IV and oral routes; the oral dose was labelled with deuterium. Besides estimation of absolute bioavailability, this design permitted assessment of metabolic clearance after IV and oral administration, and absorption could be assessed from the urinary excretion of labelled and unlabelled drug and metabolites.
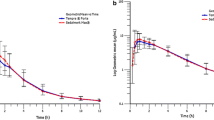
The absolute bioavailability of flecainide ranged from 79.9 to 101.1% (mean 93.6%). The absorption was 86.1 to 101.3% (mean 93.2%).
The data indicate that the variable bioavailability of flecainide is due both to metabolism and absorption. The study highlights the potential of stable isotope technique in the investigation of such issues.
Similar content being viewed by others
References
Ariens EJ (1984) Stereochemistry, a basis for sophisticated nonsense in pharmacokinetics and clinical pharmacology. Eur J Clin Pharmacol 26: 663–668
Beckmann J, Hertrampf R, Gundert-Remy U, Mikus G, Gross AS, Eichelbaum M (1988) Is there a genetic factor in flecainide toxicity? Br Med J 297: 1316
Conard GJ, Carlson GL, Frost JW, Ober RE (1979) Human plasma pharmacokinetics of flecainide-acetate (R-818) a new antiarrhythmic, following single oral and intravenous doses (abstract). Clin Pharmacol Ther 25: 218
Conard GJ, Ober RE (1984) Metabolism of flecainide. Am J Cardiol 53: 41B-51B
Eichelbaum M, Spannbrucker N, Steincke B, Dengler HJ (1979) Defective N-oxidation of sparteine in man: a new pharmacogenetic defect. Eur J Clin Pharmacol 16: 183–187
Eichelbaum M, Dengler HJ, Somogyi A, Unruh GE von (1981) Superiority of stable isotope techniques in the assessment of the bioavailability of drugs undergoing extensive first pass elimination. Eur J Clin Pharmacol 19: 127–131
Fischer C, Schönberger F, Meese CO, Eichelbaum M (1990) Determination of the enantiomers of flecainide and two major metabolites in man by gas chromatography/mass spectrometry using negative ion chemical ionisation and stable isotope labelled internal standards. Biomed Environ Mass Spectrom 19: 256–266
Fischer C, Schönberger F, Mück W, Heuck K, Eichelbaum M (1993) Simultanous assessment of the intravenous and oral disposition of the enantiomers of racemic nimodipine by chiral stationary-phase high-performance liquid chromatography and gas chromatography/mass spectrometry combined with a stable isotope technique. J Pharmaceut Sci 82: 244–250
Gross AS, Mikus G, Fischer C, Hertrampf R, Gundert-Remy U, Eichelbaum M (1989) Stereoselective disposition of flecainide in relation to the sparteine/debrisoquine metaboliser phenotype. Br J Clin Pharmacol 28: 555–566
Gross AS, Mikus G, Fischer C, Eichelbaum M (1991) Polymorphic flecainide disposition under conditions of uncontrolled urine flow and pH. Eur J Clin Pharmacol 40: 155–162
Heim M, Meyer UA (1990) Genotyping of poor metabolisers of debrisoquine by allele-specific PCR amplification. Lancet 336: 529–532
Holmes B, Heel RC (1985) Flecainide: a preliminary review of its pharmacodynamic properties and therapeutic efficacy. Drugs 29: 1–33
Kroemer HK, Turgeon J, Parker RA, Roden DM (1989) Flecainide enantiomers: disposition in human subjects and electrophysiologic actions in vitro. Clin Pharmacol Ther 46: 584–590
Mikus G, Gross AS, Beckmann J, Hertrampf R, Gundert-Remy U, Eichelbaum M (1989) The influence of the sparteine/debrisoquine phenotype on the disposition of flecainide. Clin Pharmacol Ther 45: 562–567
Roden DM, Woosley RL (1986) Flecainide. New Engl J Med 315: 36–41
Tjandra-Maga TB, Verbesselt R, Hecken A van, Mullie A, Schepper PJ de (1986) Flecainide: single and multiple oral dose kinetics, absolute bioavailability and effect of food and antacid in man. Br J Clin Pharmacol 22: 309–316
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hage, K., Fischer, C., Knebel, N.G. et al. Estimation of the absolute bioavailability of flecainide using stable isotope technique. Eur J Clin Pharmacol 48, 51–55 (1995). https://doi.org/10.1007/BF00202172
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00202172