Summary
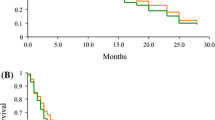
Thirty-nine previously treated patients received Nimustine in a phase II trial to test the therapeutic activity in refractory small cell lung cancer. Nimustine was given as a direct i.v. injection of 100 mg/m2 with treatments repeated every six weeks. Three partial remissions of 56, 123 and 355 days duration were noted among 34 evaluable patients. Thrombocytopenia was prominent with a median platelet nadir of 47,000/μl. We conclude that Nimustine has minor antitumor-activity in heavily pretreated patients with small cell lung cancer. The definitive value of Nimustine in the treatment of small cell lung cancer, as well as its superiority over its parent compounds remains to be established.
Similar content being viewed by others
References
Morstyn G, Ihde DC, Lichter AS: Small cell lung cancer 1973–1983: early progress and recent obstacles. J Radiation Oncol Biol Phys 10:515–539, 1984
Oldham RK, Greco FA: Small cell lung cancer. A curable disease. Cancer Chemother Pharmacol 4:173–177, 1980
Saijo N, Niitani H: Experimental and clinical effect of ACNU in Japan with emphasis on small cell carcinoma of the lung. Cancer Chemother Pharmacol 4:165–171, 1980
Nakamura T, Sasada T, Tashimo M: Mechanism of action of ACNU in leukemia cells. Cancer and Chemother 5:991–1000, 1978
Arakawa M, Shimizu F, Okada N: Effect of ACNU on leukemia L-1210. Preliminary communication. Gann 65: 191, 1974
Shimizu F, Arakawa M: Antitumor activity of ACNU in a variety of experimental tumors. Gann 69:545–548, 1978
Ogawa M: Clinical studies of new nitrosoureas under development in Japan. In: B. Serrou, PS Schein, J-L Imbach (eds): Nitrosoureas in cancer treatment. Elsevier/North-Holland Biomedical Press, 1981, pp 249–260
Broder LE, Cohen MH, Selawry OS: Treatment of bronchogenic carcinoma. II. Small cell. Cancer Treat Rev 4:219–260, 1977
WHO — Handbook for reporting results of cancer treatment. WHO Offset Publication No. 48, World Health Organization, Geneva, 1979
Author information
Authors and Affiliations
Additional information
The Swiss Group for Clinical Cancer Research (SAKK)
Rights and permissions
About this article
Cite this article
Joss, R.A., Siegenthaler, P., Ludwig, C. et al. Phase II trial of Nimustine (ACNU; 3-[(4-amino-2-methyl-5-pyrimidinyl)methyl]-1-(2-chloroethyl)-1-nitrosourea hydrochloride) in patients with small cell carcinoma of the lung after failure on combination chemotherapy. Invest New Drugs 4, 175–179 (1986). https://doi.org/10.1007/BF00194599
Issue Date:
DOI: https://doi.org/10.1007/BF00194599