Summary
Background: The new intercalative agent Mitonafide was shown in early clinical trials to be toxic to the central nervous system when administered as a short intravenous infusion, but not when given as a 120-hour continuous infusion. Thus, clinical development in different tumor types was pursued using only this administration schedule,
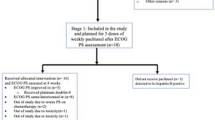
Patients and methods: Forty-nine patients with previously untreated non-small cell lung cancer(NSGLC) and at least one measurable site received Mitonafide as a 120-hour continuous (5 days) infusion every 3 weeks. The starting dose was 170 mg/m2/day × 5 in the first 26 patients and 200 mg/m2/day × 5 in the remainder. Patients were evaluated for toxicity after each course and for response every two courses and remained on treatment until excessive toxicity or disease progression were observed. A special test, the “Mini-mental state”, was used to assess patients' cognitive functions.
Results: Of the 49 patients entered, 42 were evaluable for response and toxicity. Toxicity consisted mainly of myelosuppression and no neurologic side effects were observed. Only one patient presented a partial response.
Conclusions: Although definitively safe with this schedule of administration, Mitonafide is not active in NSCLC.
Similar content being viewed by others
References
Braña MF, Castellano JM, Roldán CM: Synthesis and mode(s) of action of a new series of imide derivatives of 3-nitronaphthalic acid. Cancer Chemother Pharmacol 4:61–66, 1980
Braña MF, Martínez Sanz A, Castellano JM et al.: Synthesis and cytostatic activity of benz(de)isoquinolin-1,3-diones. Structure-activity relationships. Eur J Med Chem 16:207–212, 1981
Llombart M, Poveda A, Forner E, Fernández Martos C, Gaspar C, Muñoz M, Olmos T, Ruiz A, Soriano V, Benavides A, Martin M, Schlick E, Guillem V: Phase I study of mitonafide in solid tumors. Invest New Drugs 10:177–181, 1992
Díaz-Rubio E, Martín M, López-Vega JM, Casado A, Benavides A: Phase I study of mitonafide with a 3-day administration schedule: early interruption due to severe central nervous system toxicity. Invest New Drugs 12:277–281, 1994
Rosell R, Carles J, Abad A, Ribelles N, Barnadas A, Benavides A, Martin M: Phase I study of mitonafide in 120-hour continuous infusion in non-small cell lung cancer. Invest New Drugs 10:171–175, 1992
Folstein MF, Folstein SE, McHugh RP: “Mini-mental state”. A practical method for grading the cognitive state of patients for a clinician. J Psychiat Res 12:189–198, 1975
Miller AB, Hoogstraten B, Staquet M: Reporting results of cancer treatment. Cancer 47:207–214, 1981
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Casado, A., Rosell, R., García-Gómez, R. et al. Phase II study of mitonafide in non-small cell lung cancer (NSCLC). Invest New Drugs 14, 415–417 (1996). https://doi.org/10.1007/BF00180820
Issue Date:
DOI: https://doi.org/10.1007/BF00180820